| ⇦ | 
| ⇨ |
Atomic Radius is the distance from centre of the nucleus to the outermost shell of electrons.
[lyte id=”h6BXN0htLr4″ \]
Trends in Periodic Table
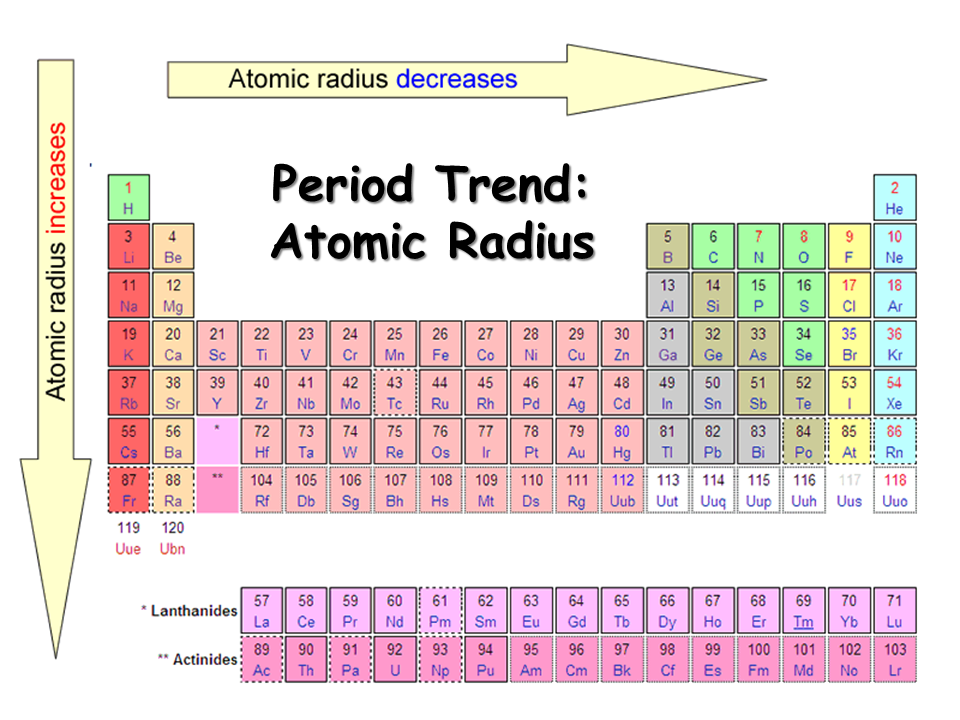
Atomic radius decreases across a period because electrons are added to the outermost shell which are at same energy level whereas protons are added to the nucleus. More number of protons in the nucleus attracts the electrons in the outer shell more strongly towards the nucleus.
Atomic radius increases moving down a group because electrons are added to new energy shells. These valance electrons experience less attraction towards the nucleus as new shell is farther away from the nucleus.
Points to Remember:
- Atomic Radius of an atom depends on the number of electrons and protons in the element.
- The fully occupied inner shells acts as shield for the electrons in the next outer shell.
- Different elements have different atomic radius depending upon the number of electrons surrounding the nucleus. Some electrons are covalent (they are shared in elements). Some electrons are ionic(lost or gained during chemical reactions). These electrons affect the radius of an atom.
- Covalent Radius is smaller than the Atomic Radius because of the overlapping of atoms in covalent bond.
Related Concepts:
Covalent Radius, Ionic Radius, Metallic Radius, Van der Wall Radius
Related Questions:
Topics: Classification of Elements and Periodicity (10)Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply