| ⇦ | 
| ⇨ |
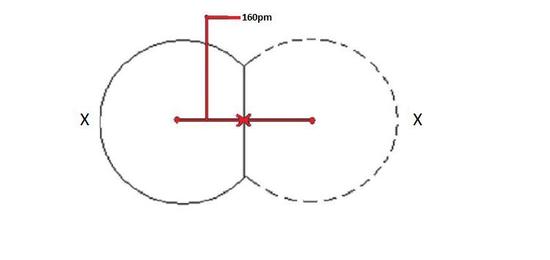
One half of the distance between nuclei of two covalently bonded atoms of same element in a molecule is called as covalent radius. Covalent radius is atomic radius of an element whose electrons are in covalent bond.
Points To Remember:
Covalent radius of an element will increase in the same pattern as that of atomic radius because bigger the radius, distance between two nucleus will also be bigger.
Topics: Classification of Elements and Periodicity (10)Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply