| ⇦ | 
| ⇨ |
A metallic radius is the measurement of an atom’s size with regards to metal elements only. One half of the distance between nuclei of two neighbouring atoms of a metal is called as metallic radii.
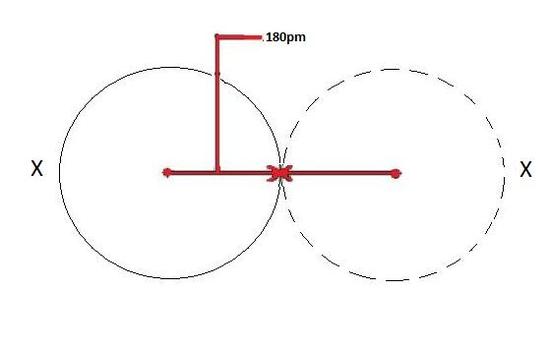
Points to Remember:
- The distance of each atom will be same because group of atoms are from the same metal.
- Metallic Radii decrease across the period because of increase in the effective nuclear charge.
- Metallic Radius increases down the group because new shell is added.
- Metallic radii are always biggest for the highest coordination number.
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply