| ⇦ | 
| ⇨ |
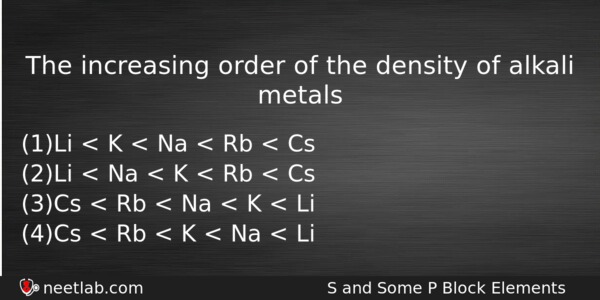
The increasing order of the density of alkali metals
Options
(a) Li < K < Na < Rb < Cs
(b) Li < Na < K < Rb < Cs
(c) Cs < Rb < Na < K < Li
(d) Cs < Rb < K < Na < Li
Correct Answer:
Li < K < Na < Rb < Cs
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Which of the following pairs show reverse properties on moving along
- In an organic compound phosphorus is estimated as
- The increasing order of atomic radius for the elements Na, Rb, K and Mg is
- At 25⁰C, the dissociation constant of a base, BOH, is 1.0 ˣ 10⁻¹².
- 40 mL of 0.1 M ammonia solution is mixed with 20 mL of 0.1 M HCl.
Topics: S and Some P Block Elements
(157)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following pairs show reverse properties on moving along
- In an organic compound phosphorus is estimated as
- The increasing order of atomic radius for the elements Na, Rb, K and Mg is
- At 25⁰C, the dissociation constant of a base, BOH, is 1.0 ˣ 10⁻¹².
- 40 mL of 0.1 M ammonia solution is mixed with 20 mL of 0.1 M HCl.
Topics: S and Some P Block Elements (157)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Li se K me K ka volume increase hota hai. Volume increase hone se
K ki density Na se bhi kam ho jati hai ( density =mass \ volume) . For this reason Li < K < Na < Rb < Cs.