| ⇦ | 
| ⇨ |
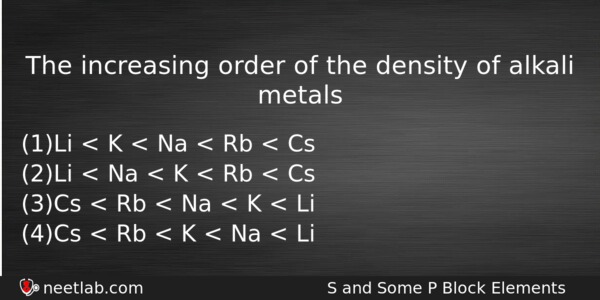
The increasing order of the density of alkali metals
Options
(a) Li < K < Na < Rb < Cs
(b) Li < Na < K < Rb < Cs
(c) Cs < Rb < Na < K < Li
(d) Cs < Rb < K < Na < Li
Correct Answer:
Li < K < Na < Rb < Cs
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Ozone is formed in the upper atmosphere from oxygen by the action of
- Strong reducing behaviour of H₃PO₂ is due to
- The mass of an object changes from 0.002 g to 0.00025 g in a time period
- Phenol reacts with bromine in chloroform at low temperature to gives
- Clemmensen reduction of a keton is carried out in the presence of
Topics: S and Some P Block Elements
(157)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Ozone is formed in the upper atmosphere from oxygen by the action of
- Strong reducing behaviour of H₃PO₂ is due to
- The mass of an object changes from 0.002 g to 0.00025 g in a time period
- Phenol reacts with bromine in chloroform at low temperature to gives
- Clemmensen reduction of a keton is carried out in the presence of
Topics: S and Some P Block Elements (157)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Li se K me K ka volume increase hota hai. Volume increase hone se
K ki density Na se bhi kam ho jati hai ( density =mass \ volume) . For this reason Li < K < Na < Rb < Cs.