| ⇦ | 
| ⇨ |
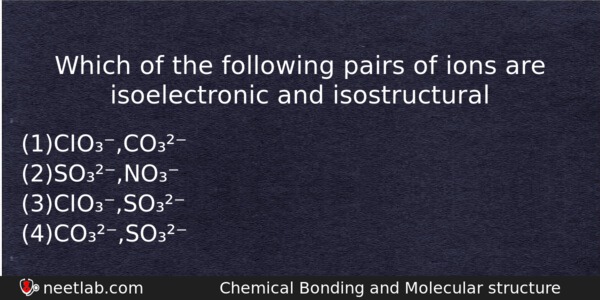
Which of the following pairs of ions are isoelectronic and isostructural
Options
(a) CIO₃⁻,CO₃²⁻
(b) SO₃²⁻,NO₃⁻
(c) CIO₃⁻,SO₃²⁻
(d) CO₃²⁻,SO₃²⁻
Correct Answer:
CIO₃⁻,SO₃²⁻
Explanation:
ClO₃⁻ and SO₃⁻ ² both have same number of electrons (42) and central atom in each being sp³ hybridised. Both are having one lone pair on central atom hence they are pyramidal.
Related Questions: - What is the volume of ’20 volume H₂O₂. required to get 5000 cm³ of oxygen at STP?
- The four bonds in methane are directed in space with an angle of
- A hydrocarbon contains 20% hydrogen and 80% carbon.The empirical formula is
- Fac-mer isomerism is associated with which one of the following complexes?
- In the extraction of copper from its sulphide ore, the metal is finally obtained
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- What is the volume of ’20 volume H₂O₂. required to get 5000 cm³ of oxygen at STP?
- The four bonds in methane are directed in space with an angle of
- A hydrocarbon contains 20% hydrogen and 80% carbon.The empirical formula is
- Fac-mer isomerism is associated with which one of the following complexes?
- In the extraction of copper from its sulphide ore, the metal is finally obtained
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply