| ⇦ | 
| ⇨ |
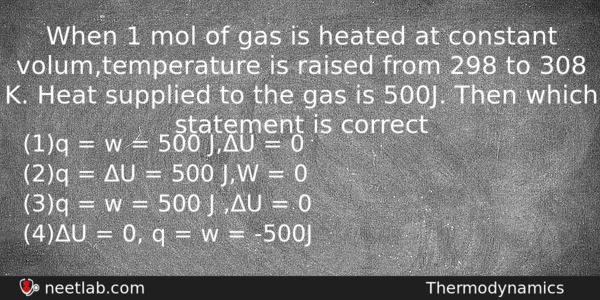
When 1 mol of gas is heated at constant volume, temperature is raised from 298 to 308 K. Heat supplied to the gas is 500J. Then which statement is correct
Options
(a) q = w = 500 J,ΔU = 0
(b) q = ΔU = 500 J,W = 0
(c) q = w = 500 J ,ΔU = 0
(d) ΔU = 0, q = w = -500J
Correct Answer:
q = ΔU = 500 J,W = 0
Explanation:
We known that ΔH = ΔE + PV
ΔH = ΔE + P Δ V + V Δ P =0
When ΔV = 0; w = 0. Therefore ΔH = ΔE + PΔV
ΔH = ΔE + 0 or ΔH = ΔE.
As ΔE = q + w , ΔE = q.
In the present problem, ΔH = 500J,
ΔV = ΔE = 500 J,q = 500 J, w = 0.
Related Questions: - When aniline is treated with sodium nitrite and hydrochloric acid at 0⁰C, it gives
- A mixture of two salts is not soluble in water but dissolves completely in dilu
- Germanium of very high purity is obtained by
- The pH of solution containing 0.10 M sodium acetate and 0.03 M acetic acid is
- Amino acids,Which build up proteins, have both the carboxylic and amino groups
Topics: Thermodynamics
(179)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- When aniline is treated with sodium nitrite and hydrochloric acid at 0⁰C, it gives
- A mixture of two salts is not soluble in water but dissolves completely in dilu
- Germanium of very high purity is obtained by
- The pH of solution containing 0.10 M sodium acetate and 0.03 M acetic acid is
- Amino acids,Which build up proteins, have both the carboxylic and amino groups
Topics: Thermodynamics (179)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply