| ⇦ | 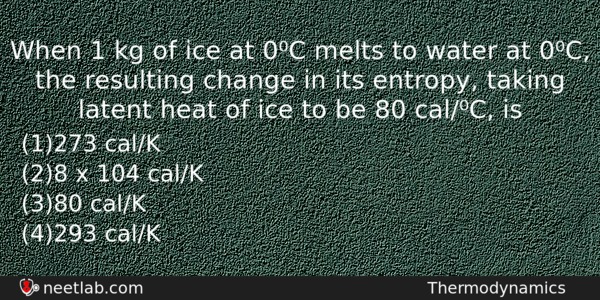
| ⇨ |
When 1 kg of ice at 0⁰C melts to water at 0⁰C, the resulting change in its entropy, taking latent heat of ice to be 80 cal/⁰C, is
Options
(a) 273 cal/K
(b) 8 x 104 cal/K
(c) 80 cal/K
(d) 293 cal/K
Correct Answer:
293 cal/K
Explanation:
Change in entropy is given by
dS = dQ / T or ∆S = ∆Q / T = mLf / 273
∆S = 1000 x 80 / 273 = 293cal / K
Related Questions: - A bullet is shot from a riffle. As a result the rifle recoils.The kinetic energy of riffle
- Which of the following is suitable for the fusion process?
- If the focal length of objective lens is increased, then magnifying power of
- The interferance pattern is obtained with two coherent light sources of intensity
- A bucket full of water is revolved in a vertical circle of 2m
Topics: Thermodynamics
(179)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A bullet is shot from a riffle. As a result the rifle recoils.The kinetic energy of riffle
- Which of the following is suitable for the fusion process?
- If the focal length of objective lens is increased, then magnifying power of
- The interferance pattern is obtained with two coherent light sources of intensity
- A bucket full of water is revolved in a vertical circle of 2m
Topics: Thermodynamics (179)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply