| ⇦ | 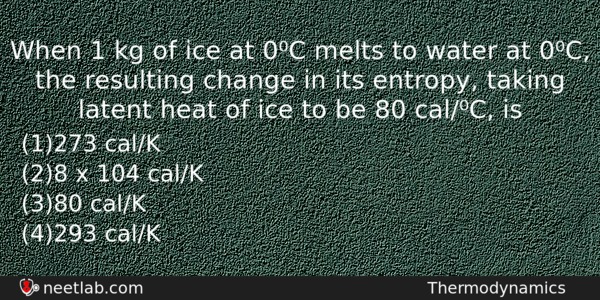
| ⇨ |
When 1 kg of ice at 0⁰C melts to water at 0⁰C, the resulting change in its entropy, taking latent heat of ice to be 80 cal/⁰C, is
Options
(a) 273 cal/K
(b) 8 x 104 cal/K
(c) 80 cal/K
(d) 293 cal/K
Correct Answer:
293 cal/K
Explanation:
Change in entropy is given by
dS = dQ / T or ∆S = ∆Q / T = mLf / 273
∆S = 1000 x 80 / 273 = 293cal / K
Related Questions: - A stone of mass 1kg is tied to a string 4m long and is rotated at constant speed
- On bombarding U²³⁵ by slow neutron, 200 MeV energy is released. If the power output
- P-type semiconductors are made by adding impurity element
- A ray of light is incident at an angle of incidence i, on one face of a prism
- The net electric force on a charge of +3μC at the mid-point on the line joining two
Topics: Thermodynamics
(179)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A stone of mass 1kg is tied to a string 4m long and is rotated at constant speed
- On bombarding U²³⁵ by slow neutron, 200 MeV energy is released. If the power output
- P-type semiconductors are made by adding impurity element
- A ray of light is incident at an angle of incidence i, on one face of a prism
- The net electric force on a charge of +3μC at the mid-point on the line joining two
Topics: Thermodynamics (179)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply