| ⇦ | 
| ⇨ |
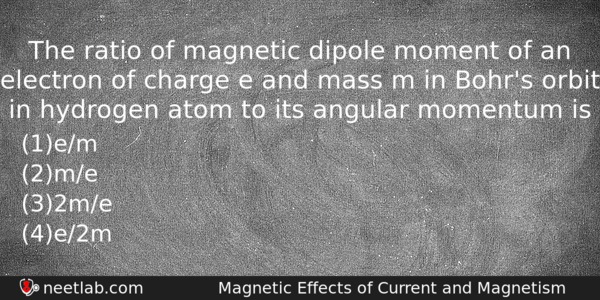
The ratio of magnetic dipole moment of an electron of charge e and mass m in Bohr’s orbit in hydrogen atom to its angular momentum is
Options
(a) e/m
(b) m/e
(c) 2m/e
(d) e/2m
Correct Answer:
e/2m
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - A person can see clearly objects only when they lie between 50 cm and 400 cm from
- The barrier potential of a p-n junction depends on
- When two displacements represented by y₁=a sin(?t) are superimposed the motion is
- The manifestation of band structure in solids is due to
- If h is Planck’s constant, the momentum of a photon of wavelength 0.01 Å is
Topics: Magnetic Effects of Current and Magnetism
(167)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A person can see clearly objects only when they lie between 50 cm and 400 cm from
- The barrier potential of a p-n junction depends on
- When two displacements represented by y₁=a sin(?t) are superimposed the motion is
- The manifestation of band structure in solids is due to
- If h is Planck’s constant, the momentum of a photon of wavelength 0.01 Å is
Topics: Magnetic Effects of Current and Magnetism (167)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Thanks