| ⇦ | 
| ⇨ |
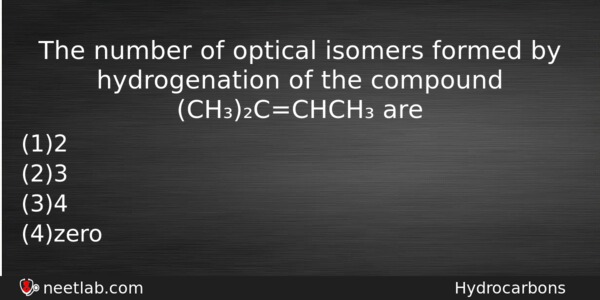
The number of optical isomers formed by hydrogenation of the compound (CH₃)₂C=CHCH₃ are
Options
(a) 2
(b) 3
(c) 4
(d) zero
Correct Answer:
zero
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Which of the following compounds will undergo racemisation when solution
- NaOH is prepared by the electrolysis of
- Among the following sets of reactants which one produces anisole
- Which of the following amino acids is basic in nature
- In which one of the following is not a buffer solution
Topics: Hydrocarbons
(84)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following compounds will undergo racemisation when solution
- NaOH is prepared by the electrolysis of
- Among the following sets of reactants which one produces anisole
- Which of the following amino acids is basic in nature
- In which one of the following is not a buffer solution
Topics: Hydrocarbons (84)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

It is because none of the carbon atoms are chiral(different substituents on the same C atom).