| ⇦ | 
| ⇨ |
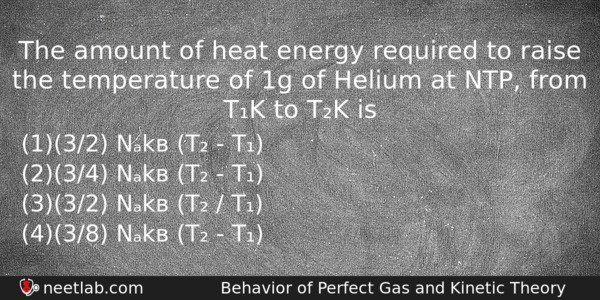
The amount of heat energy required to raise the temperature of 1g of Helium at NTP, from T₁K to T₂K is
Options
(a) (3/2) Nₐkв (T₂ – T₁)
(b) (3/4) Nₐkв (T₂ – T₁)
(c) (3/2) Nₐkв (T₂ / T₁)
(d) (3/8) Nₐkв (T₂ – T₁)
Correct Answer:
(3/8) Nₐkв (T₂ – T₁)
Explanation:
From first law of thermodynamics ∆Q = ∆U + ∆W
= (3/2) . (1/4) R (T₂ – T₁) + 0
= (3/8) Nₐkв (T₂ – T₁) [Since K = R / N ]
Related Questions: - A potentiometer wire of length L and a resistance r are connected in series with
- A person cannot see the objects clearly placed at a distance more than 40 cm
- The induced emf in a coil of 10 H inductance in which current varies
- The flux linked with a circuit is given by φ=t³+3t-7. The graph between time
- A mass of 2.0 kg is put on a flat plan attached to a vertical spring fixed on the ground
Topics: Behavior of Perfect Gas and Kinetic Theory
(34)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A potentiometer wire of length L and a resistance r are connected in series with
- A person cannot see the objects clearly placed at a distance more than 40 cm
- The induced emf in a coil of 10 H inductance in which current varies
- The flux linked with a circuit is given by φ=t³+3t-7. The graph between time
- A mass of 2.0 kg is put on a flat plan attached to a vertical spring fixed on the ground
Topics: Behavior of Perfect Gas and Kinetic Theory (34)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply