| ⇦ | 
| ⇨ |
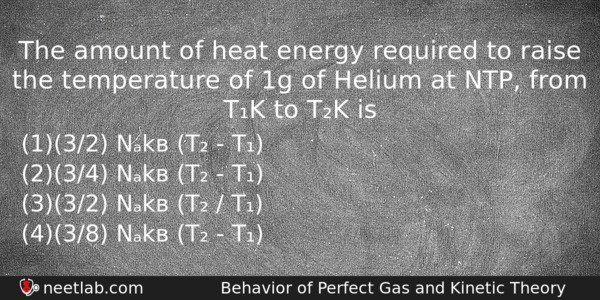
The amount of heat energy required to raise the temperature of 1g of Helium at NTP, from T₁K to T₂K is
Options
(a) (3/2) Nₐkв (T₂ – T₁)
(b) (3/4) Nₐkв (T₂ – T₁)
(c) (3/2) Nₐkв (T₂ / T₁)
(d) (3/8) Nₐkв (T₂ – T₁)
Correct Answer:
(3/8) Nₐkв (T₂ – T₁)
Explanation:
From first law of thermodynamics ∆Q = ∆U + ∆W
= (3/2) . (1/4) R (T₂ – T₁) + 0
= (3/8) Nₐkв (T₂ – T₁) [Since K = R / N ]
Related Questions: - A spherical conductor of radius 2 m is charged to a potential of 120 V. It is now
- The ionisation energy of hydrogen is 13.6 eV. The energy of the photon released
- In an AC circuit the potential differences across an inductance and resistance joined
- A heavy stone hanging from a massless string of length 15 m is projected
- What is the value of incidence L for which the current is maximum in a series LCR
Topics: Behavior of Perfect Gas and Kinetic Theory
(34)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A spherical conductor of radius 2 m is charged to a potential of 120 V. It is now
- The ionisation energy of hydrogen is 13.6 eV. The energy of the photon released
- In an AC circuit the potential differences across an inductance and resistance joined
- A heavy stone hanging from a massless string of length 15 m is projected
- What is the value of incidence L for which the current is maximum in a series LCR
Topics: Behavior of Perfect Gas and Kinetic Theory (34)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply