| ⇦ | 
| ⇨ |
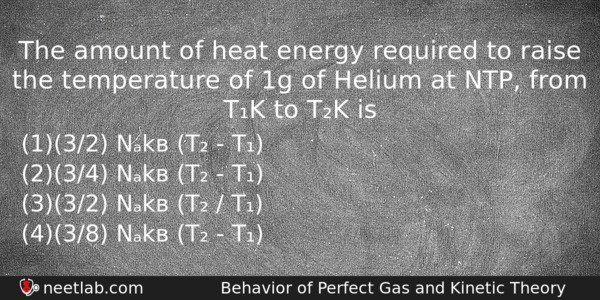
The amount of heat energy required to raise the temperature of 1g of Helium at NTP, from T₁K to T₂K is
Options
(a) (3/2) Nₐkв (T₂ – T₁)
(b) (3/4) Nₐkв (T₂ – T₁)
(c) (3/2) Nₐkв (T₂ / T₁)
(d) (3/8) Nₐkв (T₂ – T₁)
Correct Answer:
(3/8) Nₐkв (T₂ – T₁)
Explanation:
From first law of thermodynamics ∆Q = ∆U + ∆W
= (3/2) . (1/4) R (T₂ – T₁) + 0
= (3/8) Nₐkв (T₂ – T₁) [Since K = R / N ]
Related Questions: - The work function for metals A,B abd C are respectively 1.92 eV,2.0 eV and 5 eV.
- In the adiabatic compression, the decrease in volume associated with
- A block of mass 10 kg is moving in X-direction with a constant speed of 10 ms⁻¹
- Vessel A is filled with hydrogen while vessel B, whose volume is
- In photoelectric effect, the photoelectric current
Topics: Behavior of Perfect Gas and Kinetic Theory
(34)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The work function for metals A,B abd C are respectively 1.92 eV,2.0 eV and 5 eV.
- In the adiabatic compression, the decrease in volume associated with
- A block of mass 10 kg is moving in X-direction with a constant speed of 10 ms⁻¹
- Vessel A is filled with hydrogen while vessel B, whose volume is
- In photoelectric effect, the photoelectric current
Topics: Behavior of Perfect Gas and Kinetic Theory (34)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply