| ⇦ | 
| ⇨ |
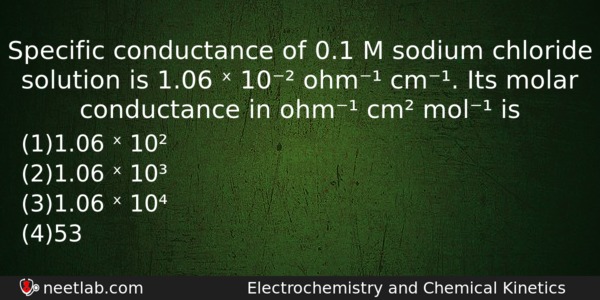
Specific conductance of 0.1 M sodium chloride solution is 1.06 ˣ 10⁻² ohm⁻¹ cm⁻¹. Its molar conductance in ohm⁻¹ cm² mol⁻¹ is
Options
(a) 1.06 ˣ 10²
(b) 1.06 ˣ 10³
(c) 1.06 ˣ 10⁴
(d) 53
Correct Answer:
1.06 ˣ 10²
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The charge on the central metal ion in the complex[Ni(CO)₄] is
- The process associated with sodium carbonate manufacture is known as….process
- CO₂ is liberated on adding sodium carbonate to a carboxylic acid.The carbon
- How many moles of iodine are liberated when 1 mole of potassium dichromate
- In the gas phase reaction C₂H₂ + H₂ ⇌ C₂H₆, the equilibrium constant
Topics: Electrochemistry and Chemical Kinetics
(87)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The charge on the central metal ion in the complex[Ni(CO)₄] is
- The process associated with sodium carbonate manufacture is known as….process
- CO₂ is liberated on adding sodium carbonate to a carboxylic acid.The carbon
- How many moles of iodine are liberated when 1 mole of potassium dichromate
- In the gas phase reaction C₂H₂ + H₂ ⇌ C₂H₆, the equilibrium constant
Topics: Electrochemistry and Chemical Kinetics (87)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply