| ⇦ | 
| ⇨ |
One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure.
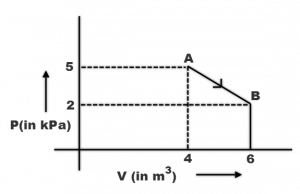
The change in internal energy of the gas during the transition is
Options
(a) -20 kJ
(b) 20 J
(c) -12 kJ
(d) 20 kJ
Correct Answer:
-20 kJ
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - If, an electron in hydrogen atom jumps from an orbit of level n=3 to an orbit
- The half life of radium is about 1600 year. Of 100 gram of radium existing now
- Angle of contact of a liquid with a solid depends on
- In a vessel, the gas is at a pressure P. If the mass of all the molecules
- In Wheatstone bridge, three resistors P,Q and R are conncected in three arms
Topics: Behavior of Perfect Gas and Kinetic Theory
(34)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If, an electron in hydrogen atom jumps from an orbit of level n=3 to an orbit
- The half life of radium is about 1600 year. Of 100 gram of radium existing now
- Angle of contact of a liquid with a solid depends on
- In a vessel, the gas is at a pressure P. If the mass of all the molecules
- In Wheatstone bridge, three resistors P,Q and R are conncected in three arms
Topics: Behavior of Perfect Gas and Kinetic Theory (34)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

ΔU = nCΔT
Also, T = PV/nR
Now,
ΔT = T-T => [PV – PV] / nR
ΔU = nR/¥-1 {PV- PV / nR} [here, ¥ = gamma]
(nR will be cancelled out)
We get,
{-8 x 10^3} / {2/5} = -20kJ.
Hope it helps 🙂