| ⇦ | 
| ⇨ |
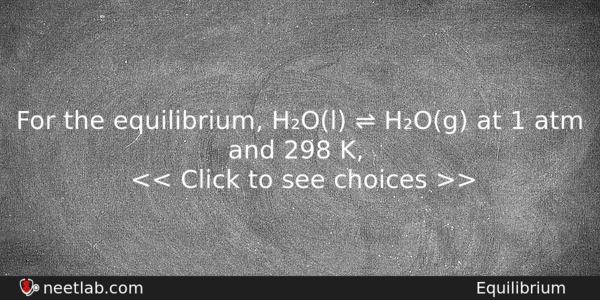
For the equilibrium, H₂O(l) ⇌ H₂O(g) at 1 atm and 298 K,
Options
(a) standard free energy change is equal to zero (ΔG⁰ = 0)
(b) free energy change is less then zero (ΔG⁰ < 0)
(c) standard free energy change is less then zero (ΔG⁰ < 0)
(d) standard free energy change is greater then zero (ΔG⁰ > 0)
Correct Answer:
free energy change is less then zero (ΔG⁰ < 0)
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The temperature above which a gas cannot be liquefied is called as
- The number of π-bond present in propyne is
- Formula of hexaaquamanganese(II)phosphate is
- Ammonia gas dissolves in water to form NH₄OH.In this reaction water acts as
- Least voltage hydrogen halide is
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The temperature above which a gas cannot be liquefied is called as
- The number of π-bond present in propyne is
- Formula of hexaaquamanganese(II)phosphate is
- Ammonia gas dissolves in water to form NH₄OH.In this reaction water acts as
- Least voltage hydrogen halide is
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply