| ⇦ | 
| ⇨ |
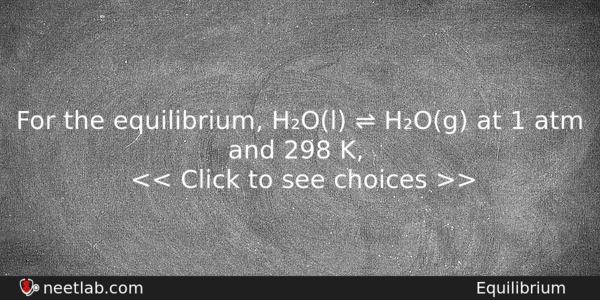
For the equilibrium, H₂O(l) ⇌ H₂O(g) at 1 atm and 298 K,
Options
(a) standard free energy change is equal to zero (ΔG⁰ = 0)
(b) free energy change is less then zero (ΔG⁰ < 0)
(c) standard free energy change is less then zero (ΔG⁰ < 0)
(d) standard free energy change is greater then zero (ΔG⁰ > 0)
Correct Answer:
free energy change is less then zero (ΔG⁰ < 0)
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - How many electrons in an atom with atomic number 105 can have (n + l) = 8
- The chemical substance used to bring down body temperature in high fever
- At 25⁰C the pH value of a solution is 6, then the solution is
- What is the mole fraction of the solute in a 1.00 m aqueous solution?
- What is the volume of ’20 volume H₂O₂. required to get 5000 cm³ of oxygen at STP?
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- How many electrons in an atom with atomic number 105 can have (n + l) = 8
- The chemical substance used to bring down body temperature in high fever
- At 25⁰C the pH value of a solution is 6, then the solution is
- What is the mole fraction of the solute in a 1.00 m aqueous solution?
- What is the volume of ’20 volume H₂O₂. required to get 5000 cm³ of oxygen at STP?
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply