Concepts
Valence Electrons
Electrons present in the outer most shell are called as Valence Electrons. They determine the chemical properties of the element.
[lyte id=”XWag7trUxeo” /]
Valence electrons of charged atoms
We know that electrons are negatively charged. […]
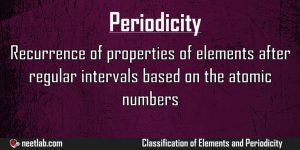
Periodicity
Recurrence of properties of elements after regular intervals based on the atomic numbers is called as periodicity. The modern periodic table is based on groups and periods and each element in a particular period exhibits similar […]
Modern Periodic Law
The modern periodic law states that physical and chemical properties of elements are periodic function of their atomic numbers.
In 1913, Moseley proved that the most fundamental property of an element is its atomic number. […]
Electron Gain Enthalpy
Electron Gain Enthalpy(EGE) is the energy released when electron is added to an isolated gaseous atom.
[lyte id=”4payRYZfWME” /]
Factors affecting Electron Gain Enthalpy: Atom Size – In smaller atoms, incoming electrons are placed closed […]
Ionization Enthalpy
Ionization Enthalpy is the energy required to remove one electron from isolated gaseous atom in ground state. It is also called as Ionisation energy or Ionisation Potential.
[lyte id=”wydOJwCrU3k” \]
Ionization Enthalpy Trends in Periodic Table […]
Ionic Radius
Effective distance from the centre of the nucleus of the ion up-to which it has an influence in the bond.
[lyte id=”vfr4qnLxaTg” \]
Ionic Radius Trends in Periodic Table When you move down the group […]
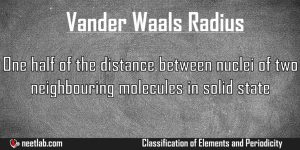
Vander Waals Radius
One half of the distance between nuclei of two neighboring molecules of the same element. When you bring two molecules of an element close to each other, then the distance between nucleus of these two molecules […]
Metallic Radius
A metallic radius is the measurement of an atom’s size with regards to metal elements only. One half of the distance between nuclei of two neighbouring atoms of a metal is called as metallic radii.