| ⇦ | 
| ⇨ |
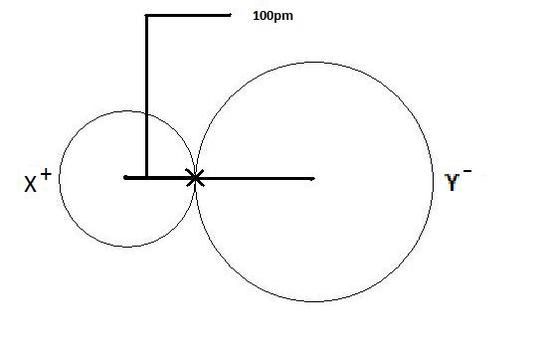
Effective distance from the centre of the nucleus of the ion up-to which it has an influence in the bond.
[lyte id=”vfr4qnLxaTg” \]
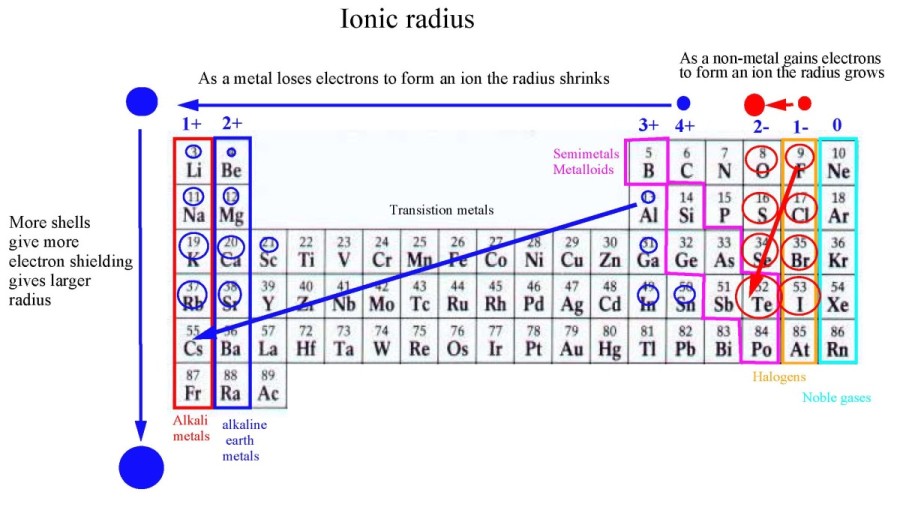
Ionic Radius Trends in Periodic Table
- When you move down the group in periodic table ionic radius increases because new shell is added.
- When you move from left to right across the period, there is no regular trend observed because each element in period can either lose or gain electron.
- Across period, the ionic radius decreases for metals because it loses an electron and becomes Cation. For non metals, ionic radius increases because it forms anions. This is because the number of electrons exceeds the number of protons when an electron is added in case of Anion.
Points to Remember
- The ionic radius will be smaller than the atomic radius, depending on the electric charge of the ion.
- The Ionic radius and the atomic radius are same for neutral atom.
- Cations have smaller ionic radius than their neutral atoms.
- Anions have bigger ionic radius than their neutral atoms.
- After comparing many compounds, Linus Pauling assigned a radius of 140 pm to O2- . This is used as the reference to determine ionic radii of other compounds.
Related Concepts:
Cation, Anion, Atomic Radius
Related Questions:
- The correct order of decreasing ionic radii among the isoelectronic species
- Which one of the following ionic species has the greatest proton affinity
- Na⁺, Mg²⁺, Al³⁺ and Si⁴⁺ are isoelectronic. The order of their ionic size is
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply