| ⇦ | 
| ⇨ |
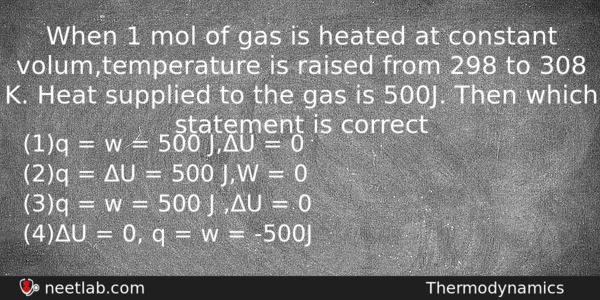
When 1 mol of gas is heated at constant volume, temperature is raised from 298 to 308 K. Heat supplied to the gas is 500J. Then which statement is correct
Options
(a) q = w = 500 J,ΔU = 0
(b) q = ΔU = 500 J,W = 0
(c) q = w = 500 J ,ΔU = 0
(d) ΔU = 0, q = w = -500J
Correct Answer:
q = ΔU = 500 J,W = 0
Explanation:
We known that ΔH = ΔE + PV
ΔH = ΔE + P Δ V + V Δ P =0
When ΔV = 0; w = 0. Therefore ΔH = ΔE + PΔV
ΔH = ΔE + 0 or ΔH = ΔE.
As ΔE = q + w , ΔE = q.
In the present problem, ΔH = 500J,
ΔV = ΔE = 500 J,q = 500 J, w = 0.
Related Questions: - Two miscible liquids can be separated by
- Reaction of diborane with ammonia gives initially
- In which following reactions aromatic aldehyde is treated with acid anhydride
- Shape of Fe(CO)₅ is
- The C-C bond length is longest in
Topics: Thermodynamics
(179)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Two miscible liquids can be separated by
- Reaction of diborane with ammonia gives initially
- In which following reactions aromatic aldehyde is treated with acid anhydride
- Shape of Fe(CO)₅ is
- The C-C bond length is longest in
Topics: Thermodynamics (179)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply