| ⇦ | 
| ⇨ |
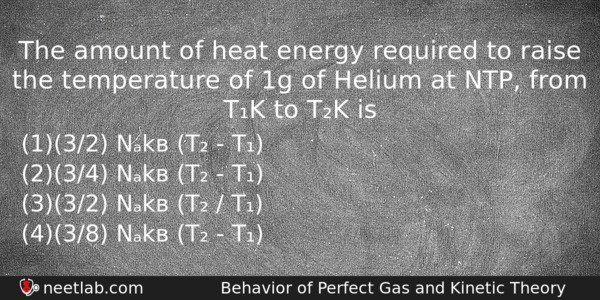
The amount of heat energy required to raise the temperature of 1g of Helium at NTP, from T₁K to T₂K is
Options
(a) (3/2) Nₐkв (T₂ – T₁)
(b) (3/4) Nₐkв (T₂ – T₁)
(c) (3/2) Nₐkв (T₂ / T₁)
(d) (3/8) Nₐkв (T₂ – T₁)
Correct Answer:
(3/8) Nₐkв (T₂ – T₁)
Explanation:
From first law of thermodynamics ∆Q = ∆U + ∆W
= (3/2) . (1/4) R (T₂ – T₁) + 0
= (3/8) Nₐkв (T₂ – T₁) [Since K = R / N ]
Related Questions: - When a beam of light is used to determine the position of an object,
- In refraction, light waves are bent on passing from one medium to the second medium
- A hollow sphere of charge does not produce an electric field at any
- The electric field part of an electromagnetic wave in a medium is represented by
- A ray of light travelling in a transparent medium of refractive index µ, falls on surface
Topics: Behavior of Perfect Gas and Kinetic Theory
(34)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- When a beam of light is used to determine the position of an object,
- In refraction, light waves are bent on passing from one medium to the second medium
- A hollow sphere of charge does not produce an electric field at any
- The electric field part of an electromagnetic wave in a medium is represented by
- A ray of light travelling in a transparent medium of refractive index µ, falls on surface
Topics: Behavior of Perfect Gas and Kinetic Theory (34)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply