Test-summary
0 of 46 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
Information
You have already completed the test before. Hence you can not start it again.
Test is loading…
You have to pass the previous Module’s test in order to start this test
Results
Results
0 of 46 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Alcohols,Phenols and Ethers 0%
- Aldehydes,Ketones and Carboxylic Acids 0%
- Biomolecules and Polymers 0%
- Chemical Bonding and Molecular Structure 0%
- Chemistry in Everyday Life 0%
- Classification of Elements and periodicity in properties 0%
- Coordination Compounds 0%
- D and F Block Elements 0%
- Electrochemistry and Chemical Kinetics 0%
- Environmental Chemistry 0%
- Equilibrium 0%
- Haloalkanes and Haloarenes 0%
- Hydrogen and Redox Reactions 0%
- Organic compounds containing Nitrogen 0%
- p-Block elements 0%
- Solid State and Solutions 0%
- Some Basic concepts of Chemistry 0%
- Some Basic Principles of organic chemistry 0%
- Structure of Atom 0%
- Surface Chemistry and Isolation of Elements 0%
- Thermodynamics 0%
-
Free Online Self-Coaching and Practice for NEET Preparation
From the above results, you can identify your weak topics and revise or relearn those topics from the NEETLab concepts, problem solving methods, revision tools, mnemonics, topic wise practice tests, mock/model test, previous NEET question papers.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- Answered
- Review
-
Question 1 of 46
1. Question
Category: Hydrogen and Redox Reactions(I) H₂O₂ + O₃ → H₂O + 2O₂
(II) H₂O₂ + Ag₂O → 2Ag + H₂O + O₂
Role of hydrogen peroxide in the above reactions is respectivelyCorrectIncorrect -
Question 2 of 46
2. Question
Category: Some Basic concepts of Chemistry1.0 g of magnesium is burnt with 0.56 g O₂ in a closed vessel.Which reactant is left in excess and how much? (At.wt.Mg=24, O=16)
CorrectIncorrect -
Question 3 of 46
3. Question
Category: p-Block elementsAcidity of diprotic acids in aqueous solutions increases in the order
CorrectIncorrect -
Question 4 of 46
4. Question
Category: Coordination CompoundsAmong the following complexes the one which shows zero crystal field stabilization energy (CFSE) is
CorrectIncorrect -
Question 5 of 46
5. Question
Category: Alcohols,Phenols and EthersAmong the following sets of reactants which one produces anisole
CorrectIncorrect -
Question 6 of 46
6. Question
Category: Chemistry in Everyday LifeArtificial sweetener which is stable under cold conditions only is
CorrectIncorrect -
Question 7 of 46
7. Question
Category: Structure of AtomBe²⁺ is isoelectronic with which of the following ions?
CorrectIncorrect -
Question 8 of 46
8. Question
Category: Structure of AtomCalculate the energy in joule corresponding to light of wavelength 45 nm.(Plank’s constant , h=6.63 ˣ 10⁻³⁴ Js, speed of light , c = 3 ˣ 10⁸ ms⁻¹)
CorrectIncorrect -
Question 9 of 46
9. Question
Category: Biomolecules and PolymersD(+)-glucose reacts with hydroxyl amine and yields an oxime. The structure of the oxime would be
CorrectIncorrect -
Question 10 of 46
10. Question
Category: Some Basic concepts of ChemistryEqual masses of H₂, O₂ and methane have been taken in a container of volume V at temperature 27⁰C in identical conditions.The ratio of the volumes of gases H₂:O₂: methane would be
CorrectIncorrect -
Question 11 of 46
11. Question
Category: EquilibriumFor a given exothermic reaction, Kp and K’p are the equilibrium constants at temperature T₁ and T₂, respectively. Assuming that heat of reaction is constant in temperature range between T₁ and T₂, it is readily observed that
CorrectIncorrect -
Question 12 of 46
12. Question
Category: ThermodynamicsFor the reaction , X₂O₄(l) → 2XO₂(g)
ΔU = 2.1 kcal, ΔS = 20 cal K⁻¹ at 300K
Hence, ΔG isCorrectIncorrect -
Question 13 of 46
13. Question
Category: EquilibriumFor the reversible reaction ,
N₂(g) + 3H₂(g) ⇌ 2NH₃(g) + heat
The equilibrium shifts in forward directionCorrectIncorrect -
Question 14 of 46
14. Question
Category: Hydrogen and Redox Reactions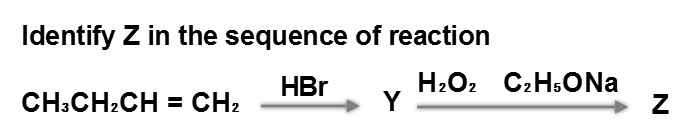 CorrectIncorrect
CorrectIncorrect -
Question 15 of 46
15. Question
Category: Solid State and SolutionsIf a is the length of the side of a cube the distance between the body centered atom and one corner atom in the cube will be
CorrectIncorrect -
Question 16 of 46
16. Question
Category: Hydrogen and Redox ReactionsIn acidic medium, H₂O₂ changes Cr₂O₇²⁻ to CrO₅ which has two ( -O – O -) bonds. Oxidation state of Cr in CrO₅ is
CorrectIncorrect -
Question 17 of 46
17. Question
Category: Organic compounds containing Nitrogen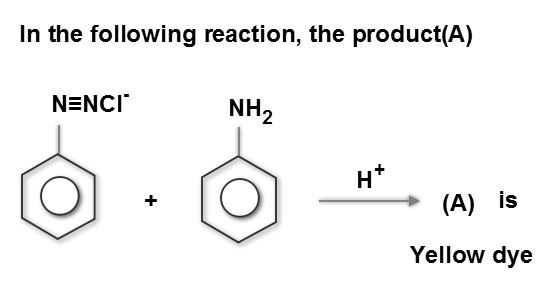 CorrectIncorrect
CorrectIncorrect -
Question 18 of 46
18. Question
Category: Some Basic Principles of organic chemistryIn the Kjeldahl’s method for estimation of nitrogen present in a soil sample, ammonia evolved from 0.75 g of sample neutralized 10 ml of 1 M H₂SO₄. The percentage of nitrogen in the soil is
CorrectIncorrect -
Question 19 of 46
19. Question
Category: D and F Block ElementsMagnetic moment 2.83 BM is given by which of the following ions
CorrectIncorrect -
Question 20 of 46
20. Question
Category: Solid State and SolutionsOf the following 0.10 m aqueous solution which one will exhibit the largest freezing point depression
CorrectIncorrect -
Question 21 of 46
21. Question
Category: D and F Block ElementsReason of lanthanoid contraction is
CorrectIncorrect -
Question 22 of 46
22. Question
Category: Hydrogen and Redox ReactionsThe pair of compounds that can exist together is
CorrectIncorrect -
Question 23 of 46
23. Question
Category: D and F Block ElementsThe reaction of aqueous KMnO₄ with H₂O₂ in acidic conditions gives
CorrectIncorrect -
Question 24 of 46
24. Question
Category: Electrochemistry and Chemical KineticsThe weight of silver (at.wt. = 108) displaced by a quantity of electricity which displaces 5600 mL of O₂ at STP will be
CorrectIncorrect -
Question 25 of 46
25. Question
Category: EquilibriumUsing the Gibb’s energy change,ΔG⁰ = +63.3 kJ, for the following reaction ,Ag₂CO₃(s) ⇌ 2Ag⁺(aq) + CO₃²⁻(aq) the Ksp of Ag₂CO₃(s) in water at 25⁰C is
CorrectIncorrect -
Question 26 of 46
26. Question
Category: Structure of AtomWhat is the maximum number of orbitals that can be identified with the following quantum numbers ? n =3,l =1,ml =0
CorrectIncorrect -
Question 27 of 46
27. Question
Category: Hydrogen and Redox ReactionsWhat products are formed when the following compound is treated with Br₂ in the presence of FeBr₃
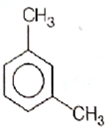 CorrectIncorrect
CorrectIncorrect -
Question 28 of 46
28. Question
Category: Electrochemistry and Chemical KineticsWhen 0.1 mol MnO₄²⁻ is oxidised the quantity of electricity required to completely oxidise MnO₄²⁻ to MnO₄⁻ is
CorrectIncorrect -
Question 29 of 46
29. Question
Category: Some Basic concepts of ChemistryWhen 22.4 litres of H₂(g) is mixed with 11.2 litres of Cl₂(g) each at S.T.P, the moles of HCl(g) formed is equal to
CorrectIncorrect -
Question 30 of 46
30. Question
Category: Coordination CompoundsWhich of the following complexes is used to be as an anticancer agent
CorrectIncorrect -
Question 31 of 46
31. Question
Category: Haloalkanes and HaloarenesWhich of the following compounds will undergo racemisation when solution of KOH hydrolyses
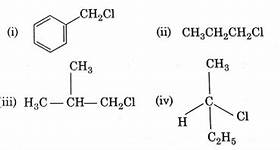 CorrectIncorrect
CorrectIncorrect -
Question 32 of 46
32. Question
Category: Biomolecules and PolymersWhich of the following hormones is produced under the conditions of stress which stimulate glycogenolysis in the liver of human beings
CorrectIncorrect -
Question 33 of 46
33. Question
Category: Chemical Bonding and Molecular StructureWhich of the following molecules has the maximum dipoles moment
CorrectIncorrect -
Question 34 of 46
34. Question
Category: Classification of Elements and periodicity in propertiesWhich of the following orders of ionic radii is correctly represented
CorrectIncorrect -
Question 35 of 46
35. Question
Category: Hydrogen and Redox ReactionsWhich of the following organic compounds has same hybridization as its combustion product (CO₂)
CorrectIncorrect -
Question 36 of 46
36. Question
Category: Biomolecules and PolymersWhich of the following organic compounds polymerizes to form the polyester Dacron
CorrectIncorrect -
Question 37 of 46
37. Question
Category: EquilibriumWhich of the following salts will give highest pH in water
CorrectIncorrect -
Question 38 of 46
38. Question
Category: Chemical Bonding and Molecular StructureWhich of the following species has plane triangular shape
CorrectIncorrect -
Question 39 of 46
39. Question
Category: ThermodynamicsWhich of the following statements is correct for the spontaneous adsorption of a gas
CorrectIncorrect -
Question 40 of 46
40. Question
Category: Organic compounds containing NitrogenWhich of the following will be most stable diazonium salt RN₂⁺ X⁻
CorrectIncorrect -
Question 41 of 46
41. Question
Category: Alcohols,Phenols and EthersWhich of the following will not be soluble in sodium hydrogen carbonate
CorrectIncorrect -
Question 42 of 46
42. Question
Category: Biomolecules and PolymersWhich one of the following is an example of thermosetting polymer
CorrectIncorrect -
Question 43 of 46
43. Question
Category: Environmental ChemistryWhich one of the following is not a Common component of photo chemical smog
CorrectIncorrect -
Question 44 of 46
44. Question
Category: Surface Chemistry and Isolation of ElementsWhich property of colloidal solution is not dependent on the charge on colloidal particles
CorrectIncorrect -
Question 45 of 46
45. Question
Category: Chemical Bonding and Molecular StructureWhich one of the following species has plane triangular shape
CorrectIncorrect -
Question 46 of 46
46. Question
Category: Environmental ChemistryWhich of the following is not a common component of Photochemical Smong
CorrectIncorrect
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply