Test-summary
0 of 45 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
Information
You have already completed the test before. Hence you can not start it again.
Test is loading…
You have to pass the previous Module’s test in order to start this test
Results
Results
0 of 45 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Alcohols,Phenols and Ethers 0%
- Aldehydes,Ketones and Carboxylic acid 0%
- Basic Concepts of Chemistry 0%
- Basic Principles of Organic Chemistry 0%
- Biomolecules and Polymers 0%
- Chemical Bonding and Molecular Structure 0%
- Chemistry in Everyday Life 0%
- Classification of Elements and Periodicity 0%
- Coordination Compounds 0%
- D and F Block Elements 0%
- Electrochemistry and Chemical Kinetics 0%
- Equilibrium 0%
- Haloalkenes and Haloarenes 0%
- Hydrocarbons 0%
- Hydrogen and Redox Reactions 0%
- Organic compounds containing Nitrogen 0%
- P Block Elements in Group 15,16,17,18 0%
- S and Some P Block Elements 0%
- Solid State and Solutions 0%
- States of Matter Gases and Liquids 0%
- Structure of Atom 0%
- Surface Chemistry and Isolation of Elements 0%
- Thermodynamics 0%
-
Free Online Self-Coaching and Practice for NEET Preparation
From the above results, you can identify your weak topics and revise or relearn those topics from the NEETLab concepts, problem solving methods, revision tools, mnemonics, topic wise practice tests, mock/model test, previous NEET question papers.
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- Answered
- Review
-
Question 1 of 45
1. Question
Category: ThermodynamicsFirst law of thermodynamics is represented by the equation
CorrectIncorrect -
Question 2 of 45
2. Question
Category: ThermodynamicsIf 1 mole of an ideal gas expands isothermally at 37⁰C from 15 litres to 25 litres ,the maximum work obtained is
CorrectIncorrect -
Question 3 of 45
3. Question
Category: EquilibriumIf the hydrogen ion concentration of an acid decreases 10 times, its pH value
CorrectIncorrect -
Question 4 of 45
4. Question
Category: EquilibriumpH value of a solution, whose hydronium ion concentration is 6.2 x 10⁻⁹ mol/L is
CorrectIncorrect -
Question 5 of 45
5. Question
Category: Hydrogen and Redox ReactionsHow many moles of iodine are liberated when 1 mole of potassium dichromate reacts with potassium iodide
CorrectIncorrect -
Question 6 of 45
6. Question
Category: Hydrogen and Redox ReactionsMnO₄²⁻ (1 mole) in neutral aqueous medium is disproportionated to
CorrectIncorrect -
Question 7 of 45
7. Question
Category: S and Some P Block ElementsNaHCO₃ is prepared by
CorrectIncorrect -
Question 8 of 45
8. Question
Category: S and Some P Block ElementsOne mole of magnesium nitride on reaction with excess of water gives
CorrectIncorrect -
Question 9 of 45
9. Question
Category: Basic Principles of Organic Chemistry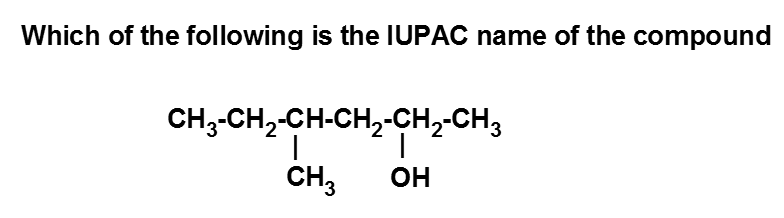 CorrectIncorrect
CorrectIncorrect -
Question 10 of 45
10. Question
Category: Basic Principles of Organic ChemistryViolet coloured complex obtained in the detection of Sulphur is
CorrectIncorrect -
Question 11 of 45
11. Question
Category: HydrocarbonsThe first fractional product of petroleum from top to bottom is
CorrectIncorrect -
Question 12 of 45
12. Question
Category: HydrocarbonsIn the commercial gasolines, the type of hydrocarbons which are more desirable is
CorrectIncorrect -
Question 13 of 45
13. Question
Category: Solid State and SolutionsWhich of the following is not a ferromagnetic substance
CorrectIncorrect -
Question 14 of 45
14. Question
Category: Solid State and SolutionsA solid compound XY has NaCl structure . If the radius of the cation is 100 pm, the radius of the anion (Y⁻) will be
CorrectIncorrect -
Question 15 of 45
15. Question
Category: Electrochemistry and Chemical KineticsMolten NaCl results in conduction because of
CorrectIncorrect -
Question 16 of 45
16. Question
Category: Electrochemistry and Chemical KineticsHow much copper is supposed to be deposited when a current of 0.75 amperes passes through a copper sulphate solution for 25 minutes
CorrectIncorrect -
Question 17 of 45
17. Question
Category: Surface Chemistry and Isolation of ElementsThe turbidity of a polymer solution measures the
CorrectIncorrect -
Question 18 of 45
18. Question
Category: Surface Chemistry and Isolation of ElementsThe catalyst used in the manufacture of H₂SO₄ by contact process is
CorrectIncorrect -
Question 19 of 45
19. Question
Category: P Block Elements in Group 15,16,17,18The solubility of I₂ increases in water in the presence of
CorrectIncorrect -
Question 20 of 45
20. Question
Category: P Block Elements in Group 15,16,17,18The oxidation number of phosphorus and basicity of acid in pyrophosphoric acid respectively are
CorrectIncorrect -
Question 21 of 45
21. Question
Category: D and F Block ElementsSodium thiosulphate is added in photography to
CorrectIncorrect -
Question 22 of 45
22. Question
Category: D and F Block ElementsThe electronic configuration of transition elements is exhibited by
CorrectIncorrect -
Question 23 of 45
23. Question
Category: Coordination CompoundsHow many edta molecules can surround calcium
CorrectIncorrect -
Question 24 of 45
24. Question
Category: Coordination CompoundsShape of Fe(CO)₅ is
CorrectIncorrect -
Question 25 of 45
25. Question
Category: Haloalkenes and HaloarenesHydrolysis of trichloromethane with aqueous KOH gives
CorrectIncorrect -
Question 26 of 45
26. Question
Category: Haloalkenes and HaloarenesTreatment of a mixture of CH₃Cl and C₂H₅Cl with sodium in dry ether can generate
CorrectIncorrect -
Question 27 of 45
27. Question
Category: Alcohols,Phenols and EthersAn isomer of ethanol is
CorrectIncorrect -
Question 28 of 45
28. Question
Category: Alcohols,Phenols and EthersGive cyclohexanol(I), acetic acid (II), 2,4,6-trinitrophenol(III), and phenol(IV).In these the order of decreasing acidic character will be
CorrectIncorrect -
Question 29 of 45
29. Question
Category: Aldehydes,Ketones and Carboxylic acidOxidation of acetaldehyde with selenium dioxide produces
CorrectIncorrect -
Question 30 of 45
30. Question
Category: Aldehydes,Ketones and Carboxylic acidAn acyl halide is formed when PCl₅ reacts with an
CorrectIncorrect -
Question 31 of 45
31. Question
Category: Organic compounds containing NitrogenThe bad smelling substance, formed by the action of alcoholic caustic potash on chloroform and aniline, is
CorrectIncorrect -
Question 32 of 45
32. Question
Category: Organic compounds containing NitrogenBest method to form aromatic iodide is
CorrectIncorrect -
Question 33 of 45
33. Question
Category: Biomolecules and PolymersEnzymes actually
CorrectIncorrect -
Question 34 of 45
34. Question
Category: Biomolecules and PolymersWhich of the following amino acids is basic in nature
CorrectIncorrect -
Question 35 of 45
35. Question
Category: Chemistry in Everyday LifeDynamite contains
CorrectIncorrect -
Question 36 of 45
36. Question
Category: States of Matter Gases and LiquidsMetallic crystalline solids have
CorrectIncorrect -
Question 37 of 45
37. Question
Category: States of Matter Gases and LiquidsIf 300 mL of a gas at 27⁰ C is cooled to 7⁰C at constant pressure, its final volume will be
CorrectIncorrect -
Question 38 of 45
38. Question
Category: Basic Concepts of ChemistryA bivalent metal has the equivalent weight of 12. The molecular weight of its oxide will be
CorrectIncorrect -
Question 39 of 45
39. Question
Category: Basic Concepts of ChemistryDalton predicted
CorrectIncorrect -
Question 40 of 45
40. Question
Category: Structure of AtomElements having same number of nucleons and different number of protons are
CorrectIncorrect -
Question 41 of 45
41. Question
Category: Structure of AtomRutherford’s experiment led to the discovery of
CorrectIncorrect -
Question 42 of 45
42. Question
Category: Classification of Elements and PeriodicityMg and Li are similar in their properties due to
CorrectIncorrect -
Question 43 of 45
43. Question
Category: Classification of Elements and PeriodicityAs the nuclear charge increases from neon to calcium the orbital energy
CorrectIncorrect -
Question 44 of 45
44. Question
Category: Chemical Bonding and Molecular StructureWhich among the following has the largest dipole moment
CorrectIncorrect -
Question 45 of 45
45. Question
Category: Chemical Bonding and Molecular StructureHe₂ does not exist because its
CorrectIncorrect
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply