| ⇦ | 
| ⇨ |
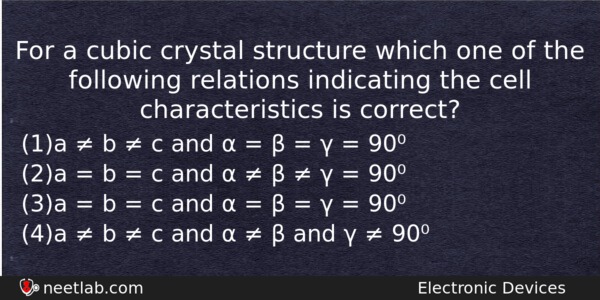
For a cubic crystal structure which one of the following relations indicating the cell characteristics is correct?
Options
(a) a ≠ b ≠ c and α = β = γ = 90⁰
(b) a = b = c and α ≠ β ≠ γ = 90⁰
(c) a = b = c and α = β = γ = 90⁰
(d) a ≠ b ≠ c and α ≠ β and γ ≠ 90⁰
Correct Answer:
a = b = c and α = β = γ = 90⁰
Explanation:
For a cubic crystal, a = b = c and α = β = γ = 90⁰
Related Questions: - A tuned amplifier circuit is used to generate a carrier frequency of 2 MHz
- In intrinsic semiconductor at room temperature number of electrons and holes are
- The innermost orbit of hydrogen atom has a diameter 1.06Å. The diameter of tenth
- In circular coil, when number of turns is doubled and resistance becomes 1/4th
- A thin circular ring of mass M and radius R rotates about an axis through its centre
Topics: Electronic Devices
(124)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A tuned amplifier circuit is used to generate a carrier frequency of 2 MHz
- In intrinsic semiconductor at room temperature number of electrons and holes are
- The innermost orbit of hydrogen atom has a diameter 1.06Å. The diameter of tenth
- In circular coil, when number of turns is doubled and resistance becomes 1/4th
- A thin circular ring of mass M and radius R rotates about an axis through its centre
Topics: Electronic Devices (124)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply