| ⇦ | 
| ⇨ |
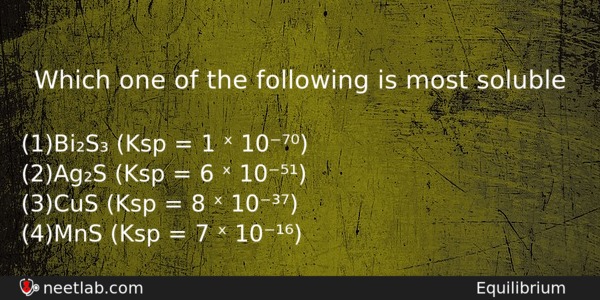
Which one of the following is most soluble
Options
(a) Bi₂S₃ (Ksp = 1 ˣ 10⁻⁷⁰)
(b) Ag₂S (Ksp = 6 ˣ 10⁻⁵¹)
(c) CuS (Ksp = 8 ˣ 10⁻³⁷)
(d) MnS (Ksp = 7 ˣ 10⁻¹⁶)
Correct Answer:
MnS (Ksp = 7 ˣ 10⁻¹⁶)
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Which of the following ions can cause coagulation of proteins
- When 22.4 litres of H₂(g) is mixed with 11.2 litres of Cl₂(g) each at S.T.P,
- ‘Metals are usually not found as nitrates in their ores”. Out of the following two
- Which of the following is correct option for free expansion of an ideal gas
- Which of the following species has four lone pairs of electrons?
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following ions can cause coagulation of proteins
- When 22.4 litres of H₂(g) is mixed with 11.2 litres of Cl₂(g) each at S.T.P,
- ‘Metals are usually not found as nitrates in their ores”. Out of the following two
- Which of the following is correct option for free expansion of an ideal gas
- Which of the following species has four lone pairs of electrons?
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply