| ⇦ | 
| ⇨ |
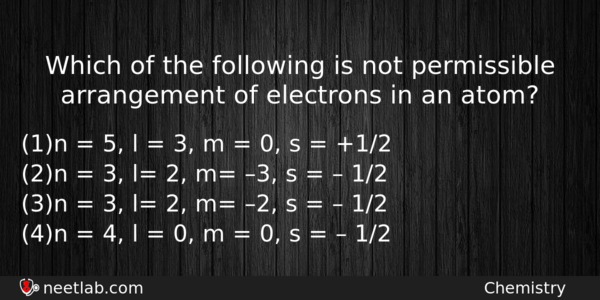
Which of the following is not permissible arrangement of electrons in an atom?
Options
(a) n = 5, l = 3, m = 0, s = +1/2
(b) n = 3, l= 2, m= –3, s = – 1/2
(c) n = 3, l= 2, m= –2, s = – 1/2
(d) n = 4, l = 0, m = 0, s = – 1/2
Correct Answer:
n = 3, l= 2, m= –3, s = – 1/2
Explanation:
m=2l+1, thus for l=2, m=5, hence values of m will be -2,-1,0,+1,+2. Therefore for l=2, m cannot have the value of -3
Related Questions: - For a first order reaction, the half -life period is independent of
- Atmosphere of big/metropolitan cities is polluted most by
- Order of reaction is decided by
- The rate constant of the reaction A → B is 0.6 ˣ 10⁻³ mol per second.
- Chronic chloroform exposure may cause damage to liver and kidney, due to the
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Structure of Atom
(90)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- For a first order reaction, the half -life period is independent of
- Atmosphere of big/metropolitan cities is polluted most by
- Order of reaction is decided by
- The rate constant of the reaction A → B is 0.6 ˣ 10⁻³ mol per second.
- Chronic chloroform exposure may cause damage to liver and kidney, due to the
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Structure of Atom (90)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply