| ⇦ | 
| ⇨ |
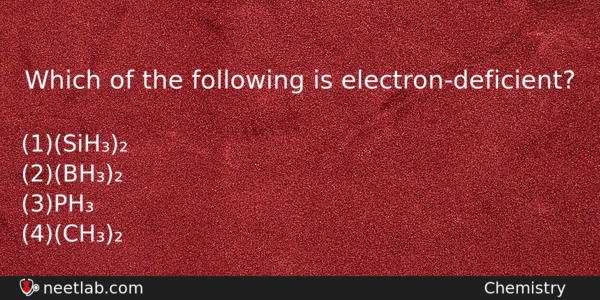
Which of the following is electron-deficient?
Options
(a) (SiH₃)₂
(b) (BH₃)₂
(c) PH₃
(d) (CH₃)₂
Correct Answer:
(BH₃)₂
Explanation:
Electron deficient molecules are compounds which do not have sufficient number of eletrons to form normal covalent bonds. (BH₃)₂ has two 3 centre – 2 electron bonds.
Related Questions: - Which of the following metals reacts with water
- Which of the following reaction is endothermic
- The structure and hybridisation of Si(CH₃)₄ is
- Aromatic hydrocarbon shows mostly
- If active mass of a 6% solution of a compound is 2, its molecular weight will be
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following metals reacts with water
- Which of the following reaction is endothermic
- The structure and hybridisation of Si(CH₃)₄ is
- Aromatic hydrocarbon shows mostly
- If active mass of a 6% solution of a compound is 2, its molecular weight will be
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply