| ⇦ | 
| ⇨ |
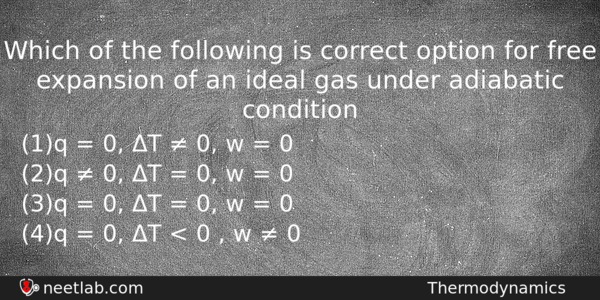
Which of the following is correct option for free expansion of an ideal gas under adiabatic condition
Options
(a) q = 0, ΔT ≠ 0, w = 0
(b) q ≠ 0, ΔT = 0, w = 0
(c) q = 0, ΔT = 0, w = 0
(d) q = 0, ΔT < 0 , w ≠ 0
Correct Answer:
q = 0, ΔT = 0, w = 0
Explanation:
For free expansion of an ideal gas under adiabatic condition, q = 0, ΔT = 0, w = 0.
Related Questions: - The main product obtained from phenol with PCl₅ is
- In N₂ + 3H₂ → 2NH₃ reversible reaction ,increases in pressure will favour
- The solubility of AgCl will be minimum in
- Principal quantum number of an atom is related to the
- certain crystals produce electric signals on application of pressur.This phenomenon
Topics: Thermodynamics
(179)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The main product obtained from phenol with PCl₅ is
- In N₂ + 3H₂ → 2NH₃ reversible reaction ,increases in pressure will favour
- The solubility of AgCl will be minimum in
- Principal quantum number of an atom is related to the
- certain crystals produce electric signals on application of pressur.This phenomenon
Topics: Thermodynamics (179)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply