| ⇦ | 
| ⇨ |
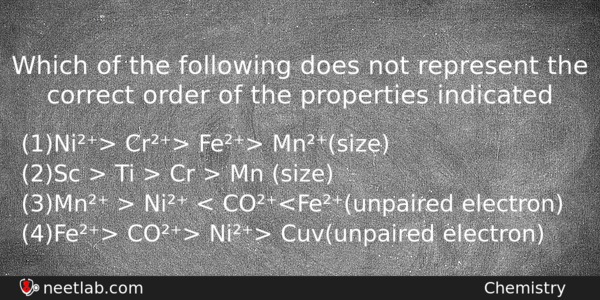
Which of the following does not represent the correct order of the properties indicated
Options
(a) Ni²⁺> Cr²⁺> Fe²⁺> Mn²⁺(size)
(b) Sc > Ti > Cr > Mn (size)
(c) Mn²⁺ > Ni²⁺ < CO²⁺
Correct Answer:
Ni²⁺> Cr²⁺> Fe²⁺> Mn²⁺(size)
Explanation:
In a period on moving left to right ionic radii decreases
Related Questions: - Which of the following is used in refrigerant
- The equilibrium weight of MnSO₄ is M/2 when it changes into
- Living in the atmosphere of CO is dangerous, because it
- One gram sample NH₄NO₃ is decomposed in a bomb calorimeter.
- In the reaction : 2P₂O₅ + 2HNO₃ → P₄O₁₀ + x, the term x is
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following is used in refrigerant
- The equilibrium weight of MnSO₄ is M/2 when it changes into
- Living in the atmosphere of CO is dangerous, because it
- One gram sample NH₄NO₃ is decomposed in a bomb calorimeter.
- In the reaction : 2P₂O₅ + 2HNO₃ → P₄O₁₀ + x, the term x is
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply