| ⇦ | 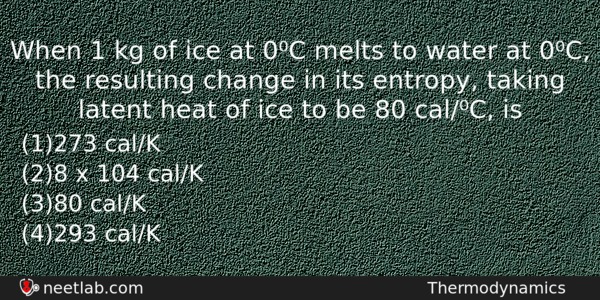
| ⇨ |
When 1 kg of ice at 0⁰C melts to water at 0⁰C, the resulting change in its entropy, taking latent heat of ice to be 80 cal/⁰C, is
Options
(a) 273 cal/K
(b) 8 x 104 cal/K
(c) 80 cal/K
(d) 293 cal/K
Correct Answer:
293 cal/K
Explanation:
Change in entropy is given by
dS = dQ / T or ∆S = ∆Q / T = mLf / 273
∆S = 1000 x 80 / 273 = 293cal / K
Related Questions: - Which of the following does not increase regularly?
- A piece of marble is projected from the earth’s surface with velocity of 50 m/s
- The current in a self-inductance L=40 mH is to be increased uniformly from 1A to 11 A
- Which of the following is the function of the step-up transformer?
- The work function of metals is in the range of 2 eV to 5 eV.
Topics: Thermodynamics
(179)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following does not increase regularly?
- A piece of marble is projected from the earth’s surface with velocity of 50 m/s
- The current in a self-inductance L=40 mH is to be increased uniformly from 1A to 11 A
- Which of the following is the function of the step-up transformer?
- The work function of metals is in the range of 2 eV to 5 eV.
Topics: Thermodynamics (179)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply