| ⇦ | 
| ⇨ |
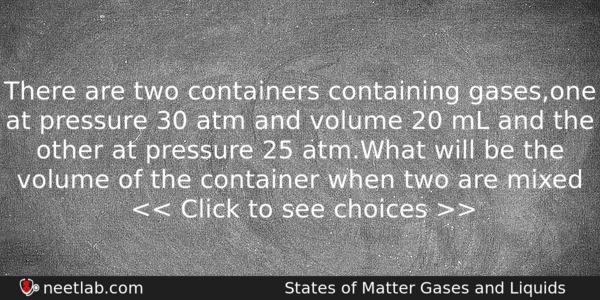
There are two containers containing gases,one at pressure 30 atm and volume 20 mL and the other at pressure 25 atm.What will be the volume of the container when two are mixed
Options
(a) 24 mL
(b) 20 mL
(c) 26 mL
(d) none of these
Correct Answer:
24 mL
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The species Ar,K⁺ and Ca²⁺ contain the same number of electrons.
- The surface tension of which of the following liquid is maximum
- The potassium ferricyanide produces on ionisation
- Which of the following statements about H₃BO₃ is not correct
- Na⁺, Mg²⁺, Al³⁺ and Si⁴⁺ are isoelectronic. The order of their ionic size is
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The species Ar,K⁺ and Ca²⁺ contain the same number of electrons.
- The surface tension of which of the following liquid is maximum
- The potassium ferricyanide produces on ionisation
- Which of the following statements about H₃BO₃ is not correct
- Na⁺, Mg²⁺, Al³⁺ and Si⁴⁺ are isoelectronic. The order of their ionic size is
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

p1v1 = p2v2
now substitute the values we will get
v2 = 24 ml
now apparently the volumes are mixed it should have gone like
v = v1 + v2
and v = 24 + 20
therefore final volume when to containers are mixed = 44 ml
P1V1=P2V2
30×20=25xV2
V2= 30×20/25
By solving
V2=24ml