| ⇦ | 
| ⇨ |
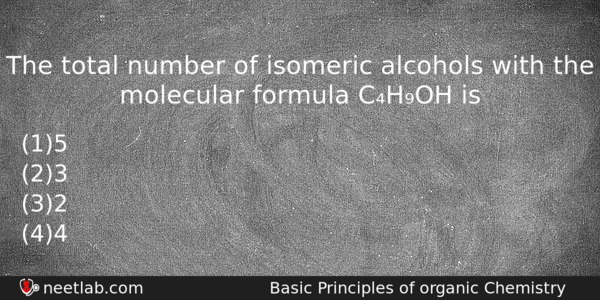
The total number of isomeric alcohols with the molecular formula C₄H₉OH is
Options
(a) 5
(b) 3
(c) 2
(d) 4
Correct Answer:
2
Explanation:
Molecular formula C₄H₉OH exists in two isomeric alcoholic forms, i.e., n-butyl alcohol and iso-butyl alcohol. therefore total number of isomeric alcohols is 2.
Related Questions: - Which one of the following reagents will be able to distinguish between 1-butyne
- All Cu(II) halides are known except the iodide. The reason for it is that
- A 6 volume sample of H₂O₂
- Set of isoelectronic species is
- In the following reaction reducing agent is
2K₃[Fe(CN)₆]+H₂O₂+2KOH→2K₄[Fe(CN)₆]
Topics: Basic Principles of Organic Chemistry
(124)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which one of the following reagents will be able to distinguish between 1-butyne
- All Cu(II) halides are known except the iodide. The reason for it is that
- A 6 volume sample of H₂O₂
- Set of isoelectronic species is
- In the following reaction reducing agent is 2K₃[Fe(CN)₆]+H₂O₂+2KOH→2K₄[Fe(CN)₆]
Topics: Basic Principles of Organic Chemistry (124)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply