| ⇦ | 
| ⇨ |
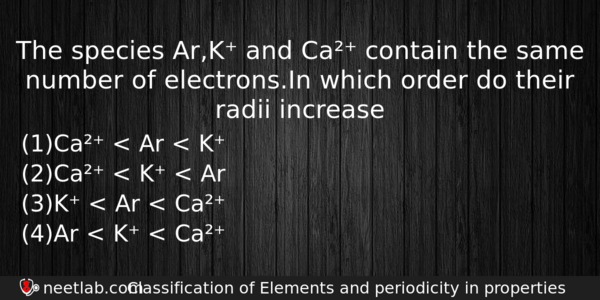
The species Ar,K⁺ and Ca²⁺ contain the same number of electrons.In which order do their radii increase
Options
(a) Ca²⁺ < Ar < K⁺
(b) Ca²⁺ < K⁺ < Ar
(c) K⁺ < Ar < Ca²⁺
(d) Ar < K⁺ < Ca²⁺
Correct Answer:
Ca²⁺ < K⁺ < Ar
Explanation:
In isoelectronic species the radius decrease with increase in nuclear charge hence increasing order of radius is Ca⁺² < K⁺< Ar
Related Questions: - Dead burnt plaster is
- An important product in the ozone depletion by chlorofluorocarbons is
- Schiff’s reagent is
- If 1,3-dibromopropane reacts with Zinc and Nal ,the product obtained is
- Solubility of M₂S salt is 3.5 ˣ 10⁻⁶ then find out solubility product
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Dead burnt plaster is
- An important product in the ozone depletion by chlorofluorocarbons is
- Schiff’s reagent is
- If 1,3-dibromopropane reacts with Zinc and Nal ,the product obtained is
- Solubility of M₂S salt is 3.5 ˣ 10⁻⁶ then find out solubility product
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply