| ⇦ | 
| ⇨ |
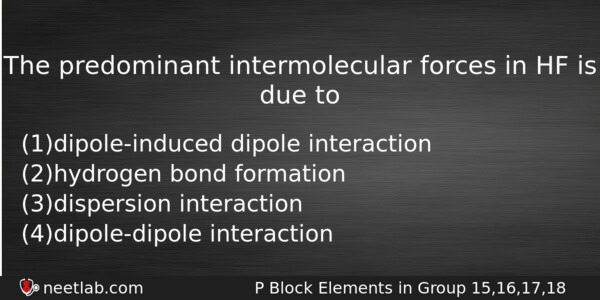
The predominant intermolecular forces in HF is due to
Options
(a) dipole-induced dipole interaction
(b) hydrogen bond formation
(c) dispersion interaction
(d) dipole-dipole interaction
Correct Answer:
hydrogen bond formation
Explanation:
In intermolecular hydrogen bonding two or more than two molecules of the same compounds combine together to give a polymeric aggregate.
Related Questions: - In steam distillation of toluene, the pressure of toluene in vapour is
- At 27⁰C average kinetic energy of one mole of helium gas can be given
- Diazo-coupling is useful to prepare some
- Two different gases enclosed in different flasks A and B at same temperature and pressure
- A hydrocarbon contains 20% hydrogen and 80% carbon.The empirical formula is
Topics: P Block Elements in Group 15
(89)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- In steam distillation of toluene, the pressure of toluene in vapour is
- At 27⁰C average kinetic energy of one mole of helium gas can be given
- Diazo-coupling is useful to prepare some
- Two different gases enclosed in different flasks A and B at same temperature and pressure
- A hydrocarbon contains 20% hydrogen and 80% carbon.The empirical formula is
Topics: P Block Elements in Group 15 (89)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply