| ⇦ | 
| ⇨ |
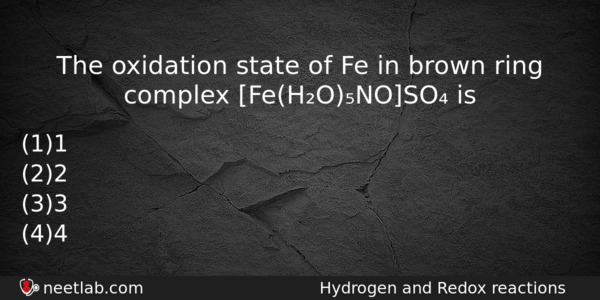
The oxidation state of Fe in brown ring complex [Fe(H₂O)₅NO]SO₄ is
Options
(a) 1
(b) 2
(c) 3
(d) 4
Correct Answer:
2
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - As we go from left to right in a period of the periodic table, gram atomic volume
- When alkyl halide is heated, with dry Ag₂O, it produces
- The crystal structure of solid Mn(II) oxide is
- The number of moles of KMnO₄ that will be needed to react with one mole of
- Which of the following alkyl halide is used as a methylating agent
Topics: Hydrogen and Redox Reactions
(174)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- As we go from left to right in a period of the periodic table, gram atomic volume
- When alkyl halide is heated, with dry Ag₂O, it produces
- The crystal structure of solid Mn(II) oxide is
- The number of moles of KMnO₄ that will be needed to react with one mole of
- Which of the following alkyl halide is used as a methylating agent
Topics: Hydrogen and Redox Reactions (174)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply