| ⇦ | 
| ⇨ |
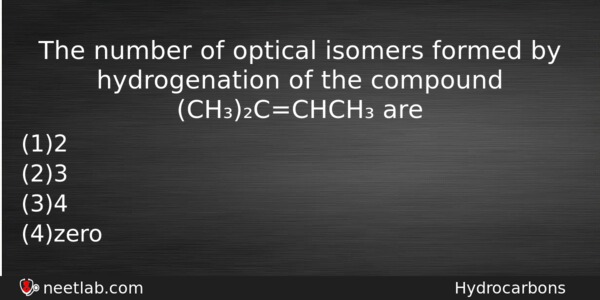
The number of optical isomers formed by hydrogenation of the compound (CH₃)₂C=CHCH₃ are
Options
(a) 2
(b) 3
(c) 4
(d) zero
Correct Answer:
zero
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - The maximum number of molecules is present in
- For a first order reaction, the half -life period is independent of
- The oxidation state of I in H₄IO₆⁻ is
- The preparation of ethene from ethanol can be described as
- If Nₐ is Avagadro number then number of valence electrons in 4.2g of nitride
Topics: Hydrocarbons
(84)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The maximum number of molecules is present in
- For a first order reaction, the half -life period is independent of
- The oxidation state of I in H₄IO₆⁻ is
- The preparation of ethene from ethanol can be described as
- If Nₐ is Avagadro number then number of valence electrons in 4.2g of nitride
Topics: Hydrocarbons (84)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

It is because none of the carbon atoms are chiral(different substituents on the same C atom).