| ⇦ | 
| ⇨ |
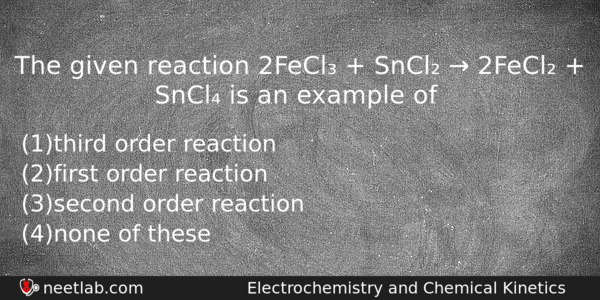
The given reaction 2FeCl₃ + SnCl₂ → 2FeCl₂ + SnCl₄ is an example of
Options
(a) third order reaction
(b) first order reaction
(c) second order reaction
(d) none of these
Correct Answer:
third order reaction
Explanation:
For a general reaction xA + yB + zC → product, the order of reaction is x + y + z. since three molecules undergo change in concentration, therefore it is a third order reaction.
Related Questions: - Which one of the following is not an amphoteric substance
- Benzaldehyde gives a position test with
- Which of the suggested tests can be used to differentiate the given compounds
- Which of the following is a redox reaction
- Crystalline solids are
Topics: Electrochemistry and Chemical Kinetics
(87)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which one of the following is not an amphoteric substance
- Benzaldehyde gives a position test with
- Which of the suggested tests can be used to differentiate the given compounds
- Which of the following is a redox reaction
- Crystalline solids are
Topics: Electrochemistry and Chemical Kinetics (87)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply