| ⇦ | 
| ⇨ |
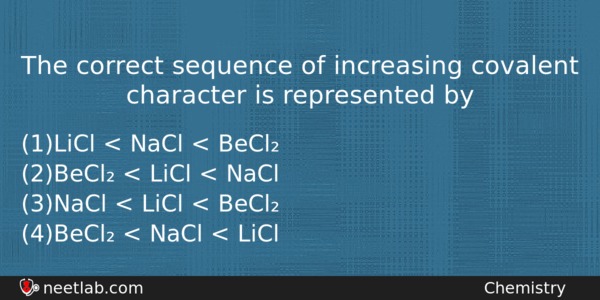
The correct sequence of increasing covalent character is represented by
Options
(a) LiCl < NaCl < BeCl₂
(b) BeCl₂ < LiCl < NaCl
(c) NaCl < LiCl < BeCl₂
(d) BeCl₂ < NaCl < LiCl
Correct Answer:
NaCl < LiCl < BeCl₂
Explanation:
As difference of electronegativity increases % ionic character increases and covalentcharacter decreases i.e. negativity differences decreases covalent character increases.
Further greater the charge on the cation more will be its covalent character.Be has maximum (+2) charge.
Related Questions: - A particular solid is very hard and has a very high melting point. In solid
- Reaction of phenol with chloroform in presence of dilute sodium hydroxide finally
- Benzene hexachloride is an
- NaOCl is used as a bleaching agent and sterilising agent. It can be synthesized
- The standard emf for the cell reaction, 2Cu⁺(aq) → Cu(s) + Cu²⁺(aq)
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A particular solid is very hard and has a very high melting point. In solid
- Reaction of phenol with chloroform in presence of dilute sodium hydroxide finally
- Benzene hexachloride is an
- NaOCl is used as a bleaching agent and sterilising agent. It can be synthesized
- The standard emf for the cell reaction, 2Cu⁺(aq) → Cu(s) + Cu²⁺(aq)
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply