| ⇦ | 
| ⇨ |
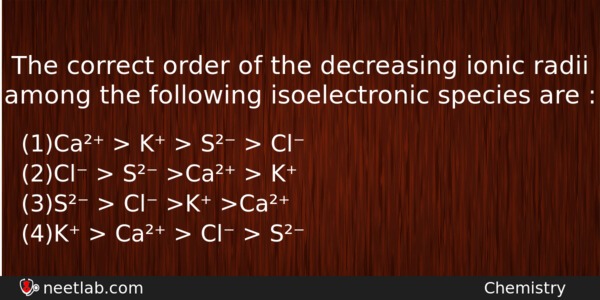
The correct order of the decreasing ionic radii among the following isoelectronic species are :
Options
(a) Ca²⁺ > K⁺ > S²⁻ > Cl⁻
(b) Cl⁻ > S²⁻ >Ca²⁺ > K⁺
(c) S²⁻ > Cl⁻ >K⁺ >Ca²⁺
(d) K⁺ > Ca²⁺ > Cl⁻ > S²⁻
Correct Answer:
S²⁻ > Cl⁻ >K⁺ >Ca²⁺
Explanation:
Among the isoelectronic species, size increases with the increase in negative charge. Thus S has the highest negative charge and hence largest in size followed by Cl, K and Ca
Related Questions: - The rate of reactions exhibiting negative activation energy
- The electrophile in the nitration of benzene is
- CN⁻ is a strong fields ligand.This is due to the fact that
- Bhor’s theory is applicable to
- What will be the correct relationship between free energy and equilibrium constant K
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The rate of reactions exhibiting negative activation energy
- The electrophile in the nitration of benzene is
- CN⁻ is a strong fields ligand.This is due to the fact that
- Bhor’s theory is applicable to
- What will be the correct relationship between free energy and equilibrium constant K
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply