| ⇦ | 
| ⇨ |
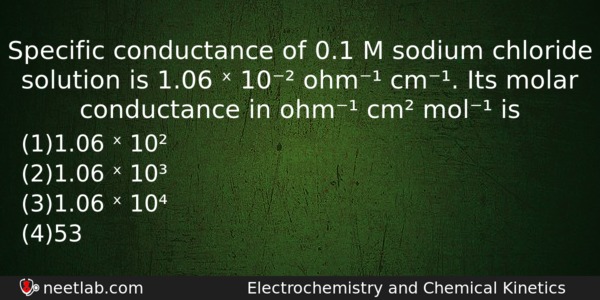
Specific conductance of 0.1 M sodium chloride solution is 1.06 ˣ 10⁻² ohm⁻¹ cm⁻¹. Its molar conductance in ohm⁻¹ cm² mol⁻¹ is
Options
(a) 1.06 ˣ 10²
(b) 1.06 ˣ 10³
(c) 1.06 ˣ 10⁴
(d) 53
Correct Answer:
1.06 ˣ 10²
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - When carbon monoxide is passed over solid caustic soda heated to 200▫ C it forms
- Ethyne can be oxidised to oxalic acid by using
- The oxidation numbers of hydrogen in KH,MgH₂ and NaH are respectively
- Bauxite is concentrated by
- In acidic medium, the equivalent weight of KMnO₄ is
Topics: Electrochemistry and Chemical Kinetics
(87)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- When carbon monoxide is passed over solid caustic soda heated to 200▫ C it forms
- Ethyne can be oxidised to oxalic acid by using
- The oxidation numbers of hydrogen in KH,MgH₂ and NaH are respectively
- Bauxite is concentrated by
- In acidic medium, the equivalent weight of KMnO₄ is
Topics: Electrochemistry and Chemical Kinetics (87)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply