| ⇦ | 
| ⇨ |
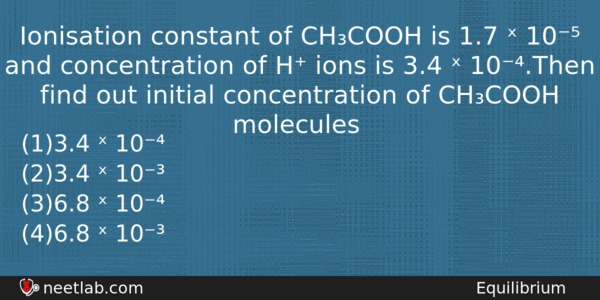
Ionisation constant of CH₃COOH is 1.7 ˣ 10⁻⁵ and concentration of H⁺ ions is 3.4 ˣ 10⁻⁴.Then find out initial concentration of CH₃COOH molecules
Options
(a) 3.4 ˣ 10⁻⁴
(b) 3.4 ˣ 10⁻³
(c) 6.8 ˣ 10⁻⁴
(d) 6.8 ˣ 10⁻³
Correct Answer:
6.8 ˣ 10⁻³
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - If 1 litre of N₂ is mixed with 2 litre of O₂, quantity explaining it is
- Which of the following is the green coloured powder produced when ammonium
- Anomalous behaviour of nitrogen is due to
- The solubility of I₂ increases in water in the presence of
- If pH of a saturated solution of Ba(OH)₂ is 12, the value of its Ksp is
Topics: Equilibrium
(104)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If 1 litre of N₂ is mixed with 2 litre of O₂, quantity explaining it is
- Which of the following is the green coloured powder produced when ammonium
- Anomalous behaviour of nitrogen is due to
- The solubility of I₂ increases in water in the presence of
- If pH of a saturated solution of Ba(OH)₂ is 12, the value of its Ksp is
Topics: Equilibrium (104)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply