| ⇦ | 
| ⇨ |
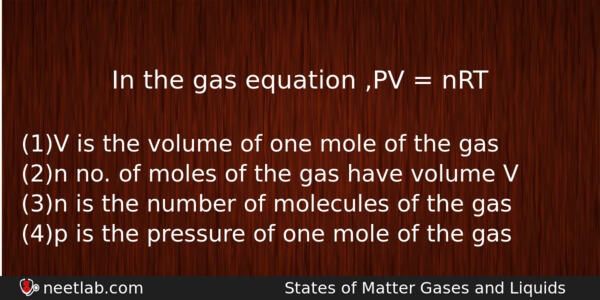
In the gas equation ,PV = nRT
Options
(a) V is the volume of one mole of the gas
(b) n no. of moles of the gas have volume V
(c) n is the number of molecules of the gas
(d) p is the pressure of one mole of the gas
Correct Answer:
n is the number of molecules of the gas
Explanation:
Ideal gas equation is : PV = nRT. P = Pressure of the gas. n = no.of molecules of gas . T = temperature. V = volume of gas. R = gas constant
Related Questions: - Which of the following processes does not involve oxidation of iron
- Which of the following molecules has the largest root mean square velocity at 25°C
- For the reaction R → P a graph of [R] against time is found to be a straight
- Which of the following lanthanide is commonly used
- Different gases at the same temperature must have
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following processes does not involve oxidation of iron
- Which of the following molecules has the largest root mean square velocity at 25°C
- For the reaction R → P a graph of [R] against time is found to be a straight
- Which of the following lanthanide is commonly used
- Different gases at the same temperature must have
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply