| ⇦ | 
| ⇨ |
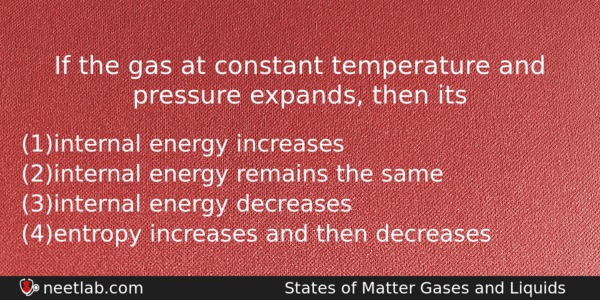
If the gas at constant temperature and pressure expands, then its
Options
(a) internal energy increases
(b) internal energy remains the same
(c) internal energy decreases
(d) entropy increases and then decreases
Correct Answer:
internal energy remains the same
Explanation:
For an ideal gas, the internal energy depends upon temperature. As expansion takes place at constant temperature and pressure so internal energy of the gas remains constant, i.e. ΔE = 0.
Related Questions: - Which of the following statements is correct
- The oxyacid of sulphur that contains a lone pair of electrons on sulphur is
- Magnetic quantum number specifies
- Rectified spirit has ethanol
- The ability of a given substance to assume two or more crystalline structure is
Topics: States of Matter Gases and Liquids
(80)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Which of the following statements is correct
- The oxyacid of sulphur that contains a lone pair of electrons on sulphur is
- Magnetic quantum number specifies
- Rectified spirit has ethanol
- The ability of a given substance to assume two or more crystalline structure is
Topics: States of Matter Gases and Liquids (80)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Internal energy at any state is a function of number of moles n and the temperature. If the the volume expansion takes place at constant temperature and pressure we see that using the gas equation PV=nRT, we get V∝n and thus the number of moles of gas are increasing(may be the gas is being introduces by an external agent), thus internal energy is increasing.
The internal energy remains same because internal energy directly depends upon temperatures and the temp is constant so internal energy also remains same