| ⇦ | 
| ⇨ |
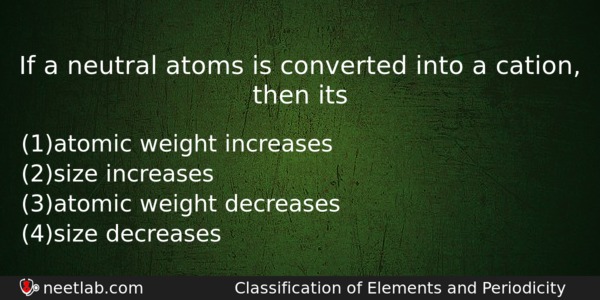
If a neutral atoms is converted into a cation, then its
Options
(a) atomic weight increases
(b) size increases
(c) atomic weight decreases
(d) size decreases
Correct Answer:
size decreases
Explanation:
After the removal of electron, to form cation, there is more effective nuclear charge on remaining electrons in the cation, so size decreases.
Related Questions: - Terfenadine is commonly used as a
- In third group, iron gives blood red colouration with ammonium thiocyanate
- According to law of mass action rate of a chemical reaction is proportional to
- Which of the following has ability to release bromine from potassium bromide
- Formation of methyl tertiary butyl ether by the reaction of sodium tertiary
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Terfenadine is commonly used as a
- In third group, iron gives blood red colouration with ammonium thiocyanate
- According to law of mass action rate of a chemical reaction is proportional to
- Which of the following has ability to release bromine from potassium bromide
- Formation of methyl tertiary butyl ether by the reaction of sodium tertiary
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply