| ⇦ | 
| ⇨ |
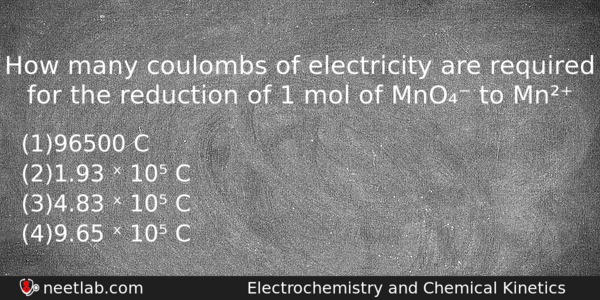
How many coulombs of electricity are required for the reduction of 1 mol of MnO₄⁻ to Mn²⁺
Options
(a) 96500 C
(b) 1.93 ˣ 10⁵ C
(c) 4.83 ˣ 10⁵ C
(d) 9.65 ˣ 10⁵ C
Correct Answer:
4.83 ˣ 10⁵ C
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - If Ksp for HgSO₄ is 6.4 x 10⁻⁵, then solubility of the salt is
- Surface water contains
- Choose isosteres from the following.
- A sugar that is not a disaccharide among the following is
- Which of these have no unit?
Topics: Electrochemistry and Chemical Kinetics
(87)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- If Ksp for HgSO₄ is 6.4 x 10⁻⁵, then solubility of the salt is
- Surface water contains
- Choose isosteres from the following.
- A sugar that is not a disaccharide among the following is
- Which of these have no unit?
Topics: Electrochemistry and Chemical Kinetics (87)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply