| ⇦ | 
| ⇨ |
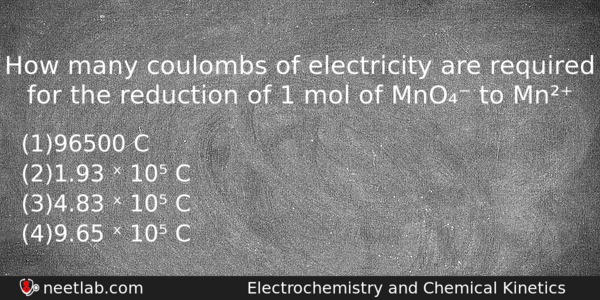
How many coulombs of electricity are required for the reduction of 1 mol of MnO₄⁻ to Mn²⁺
Options
(a) 96500 C
(b) 1.93 ˣ 10⁵ C
(c) 4.83 ˣ 10⁵ C
(d) 9.65 ˣ 10⁵ C
Correct Answer:
4.83 ˣ 10⁵ C
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - On heating glycerol with conc.H₂SO₄, a compound is obtained which has bad odour.
- The number of water molecules is maximum in
- Which of the following statements is not correct
- The set of quantom number for 19th electrons of chromium(Z=24) is
- If 30 mL of H₂ and 20 mL of O₂ reacts to form water. what is left at the end
Topics: Electrochemistry and Chemical Kinetics
(87)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- On heating glycerol with conc.H₂SO₄, a compound is obtained which has bad odour.
- The number of water molecules is maximum in
- Which of the following statements is not correct
- The set of quantom number for 19th electrons of chromium(Z=24) is
- If 30 mL of H₂ and 20 mL of O₂ reacts to form water. what is left at the end
Topics: Electrochemistry and Chemical Kinetics (87)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply