| ⇦ | 
| ⇨ |
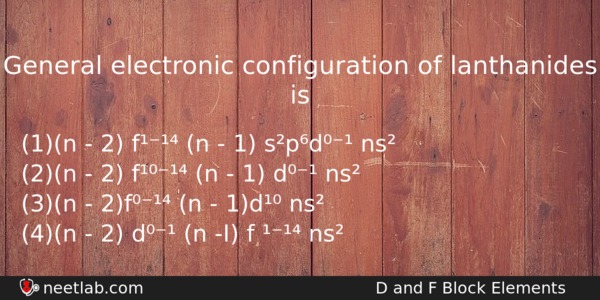
General electronic configuration of lanthanides is
Options
(a) (n – 2) f¹⁻¹⁴ (n – 1) s²p⁶d⁰⁻¹ ns²
(b) (n – 2) f¹⁰⁻¹⁴ (n – 1) d⁰⁻¹ ns²
(c) (n – 2)f⁰⁻¹⁴ (n – 1)d¹⁰ ns²
(d) (n – 2) d⁰⁻¹ (n -I) f ¹⁻¹⁴ ns²
Correct Answer:
(n – 2) f¹⁻¹⁴ (n – 1) s²p⁶d⁰⁻¹ ns²
Explanation:
Lanthanides undergo Aufbau’s principle. The principle states that the electrons should be filled in the ground state first, next the excited state will be filled, so have lanthanide comes under F-block elements, Here 14 electrons can be filled from lowest energy levels to highest energy level. The general electronic configuration is (n-2) f⁽¹⁻¹⁴⁾ (n-1) s²p⁶d⁰⁻¹ n s².
Related Questions: - Addition of phosphate fertilizers into water leads to
- An ion has 18 electrons in the outermost shell, it is
- An ester used as medicine is
- The number of moles of KMnO₄ reduced by one mole of KI in alkaline medium is
- The number of significant figures for the three numbers 161 cm, 0.161 cm
Topics: D and F Block Elements
(91)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Addition of phosphate fertilizers into water leads to
- An ion has 18 electrons in the outermost shell, it is
- An ester used as medicine is
- The number of moles of KMnO₄ reduced by one mole of KI in alkaline medium is
- The number of significant figures for the three numbers 161 cm, 0.161 cm
Topics: D and F Block Elements (91)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

According to Aufbau principal, firstly electrons must be filled in lower energy level. Lanthanides have electronic configuration [Xe] 4f^0-14 5d^0-1 6s^2
Energy order is 6s<4f~5d