| ⇦ | 
| ⇨ |
Figure below shows two paths that may be taken by a gas to go from a state A to a state C. In process AB, 400 J of heat is added to the system and in process BC, 100 J of heat is added to the system. The heat absorbed by the system in the process AC will be
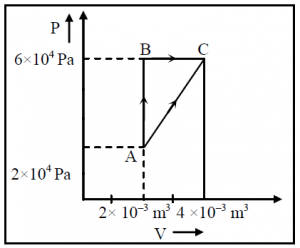
Options
(a) 500 J
(b) 460 J
(c) 300 J
(d) 380 J
Correct Answer:
460 J
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - A wave travelling in the +ve x-direction having displacement along y-direction as 1m
- An electron moves on a straight line path XY as shown. The abcd is a coil adjacent
- A body of mass m=3.513 kg is moving along the x-axis with a speed of 5 m/s
- The ratio of the acceleration for a solid sphere(mass m and radius R)rolling down
- A rocket is fired upward from the earth surface such that it creates an acceleration
Topics: Thermodynamics
(179)
Subject: Physics
(2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A wave travelling in the +ve x-direction having displacement along y-direction as 1m
- An electron moves on a straight line path XY as shown. The abcd is a coil adjacent
- A body of mass m=3.513 kg is moving along the x-axis with a speed of 5 m/s
- The ratio of the acceleration for a solid sphere(mass m and radius R)rolling down
- A rocket is fired upward from the earth surface such that it creates an acceleration
Topics: Thermodynamics (179)
Subject: Physics (2479)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply