| ⇦ | 
| ⇨ |
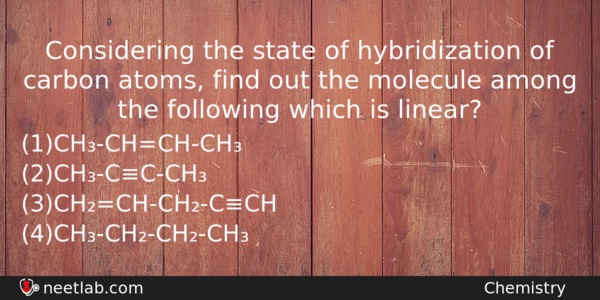
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear?
Options
(a) CH₃-CH=CH-CH₃
(b) CH₃-C≡C-CH₃
(c) CH₂=CH-CH₂-C≡CH
(d) CH₃-CH₂-CH₂-CH₃
Correct Answer:
CH₃-C≡C-CH₃
Explanation:
sp³ sp sp sp³
H₃C – C ≡ C – CH₃
linear
Related Questions: - The volume of water to be added to N/2 HCl to prepare 500 cm³ of N/10 solution is
- Alkyl halides can be converted into alcohol in a single step reaction.
- The cylindrical shape of an alkyne is due to
- Which one is not a constituent of nucleic acid
- The correct order of ionization energy of C, N, O, F is
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- The volume of water to be added to N/2 HCl to prepare 500 cm³ of N/10 solution is
- Alkyl halides can be converted into alcohol in a single step reaction.
- The cylindrical shape of an alkyne is due to
- Which one is not a constituent of nucleic acid
- The correct order of ionization energy of C, N, O, F is
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply