| ⇦ | 
| ⇨ |
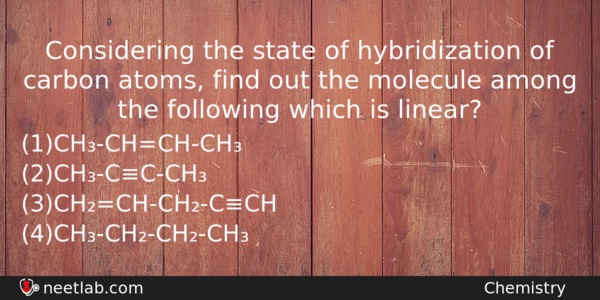
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear?
Options
(a) CH₃-CH=CH-CH₃
(b) CH₃-C≡C-CH₃
(c) CH₂=CH-CH₂-C≡CH
(d) CH₃-CH₂-CH₂-CH₃
Correct Answer:
CH₃-C≡C-CH₃
Explanation:
sp³ sp sp sp³
H₃C – C ≡ C – CH₃
linear
Related Questions: - Fish die in water bodies polluted by sewage due to
- If a 0.00001 M solution of HCl is diluted thousand folds the pH of the resulting
- For the cell reactionCu²⁺ (C₁.aq) + Zn(s) = Zn²⁺(C₂.aq ) + Cu(s)
- Acetamide reacts with NaOBr in the alkaline medium to form
- Which of the following statement about water is wrong?
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Chemical Bonding and Molecular Structure
(86)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Fish die in water bodies polluted by sewage due to
- If a 0.00001 M solution of HCl is diluted thousand folds the pH of the resulting
- For the cell reactionCu²⁺ (C₁.aq) + Zn(s) = Zn²⁺(C₂.aq ) + Cu(s)
- Acetamide reacts with NaOBr in the alkaline medium to form
- Which of the following statement about water is wrong?
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Chemical Bonding and Molecular Structure (86)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply