| ⇦ | 
| ⇨ |
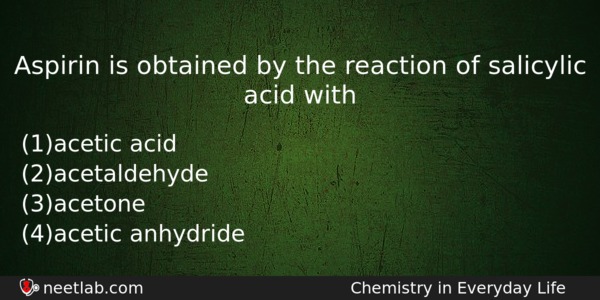
Aspirin is obtained by the reaction of salicylic acid with
Options
(a) acetic acid
(b) acetaldehyde
(c) acetone
(d) acetic anhydride
Correct Answer:
acetic anhydride
Explanation:
The aspirin is obtained by the reaction of salicylic acid with acetyl chloride. It is used as an analgesic to relieve body pain.
Related Questions: - A solution with pH = 2 is more acidic then one with a pH = 6 , by a factor
- Correct order of the stability of group IIA metal carbonates is
- The polymer used in orthopaedic devices and in controlled drug release is
- Correct order of first IP among following elements Be, B, C, N, O is
- How much copper is supposed to be deposited when a current of 0.75 amperes
Topics: Chemistry in Everyday Life
(71)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- A solution with pH = 2 is more acidic then one with a pH = 6 , by a factor
- Correct order of the stability of group IIA metal carbonates is
- The polymer used in orthopaedic devices and in controlled drug release is
- Correct order of first IP among following elements Be, B, C, N, O is
- How much copper is supposed to be deposited when a current of 0.75 amperes
Topics: Chemistry in Everyday Life (71)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply