| ⇦ | 
| ⇨ |
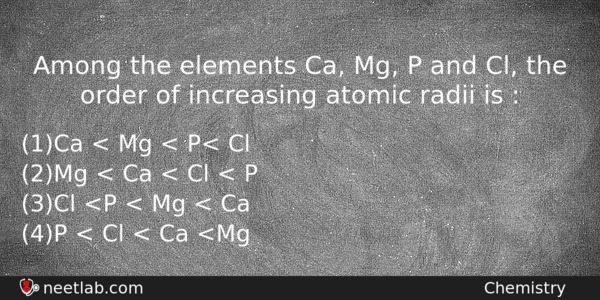
Among the elements Ca, Mg, P and Cl, the order of increasing atomic radii is :
Options
(a) Ca < Mg < P< Cl
(b) Mg < Ca < Cl < P
(c) Cl
Correct Answer:
Cl
Explanation:
No explanation available. Be the first to write the explanation for this question by commenting below.
Related Questions: - Hereditary characteristic are passed on from parents to chlidren through
- At higher temperature,iodoform reaction is given by
- The interatomic distances in H₂ and Cl molecules are 74 and 198 pm respectively
- Carbylamine reaction is given by
- Which of the following is a disproportionation reaction
Question Type: Memory
(964)
Difficulty Level: Easy
(1008)
Topics: Classification of Elements and Periodicity
(94)
Subject: Chemistry
(2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
- Hereditary characteristic are passed on from parents to chlidren through
- At higher temperature,iodoform reaction is given by
- The interatomic distances in H₂ and Cl molecules are 74 and 198 pm respectively
- Carbylamine reaction is given by
- Which of the following is a disproportionation reaction
Question Type: Memory (964)
Difficulty Level: Easy (1008)
Topics: Classification of Elements and Periodicity (94)
Subject: Chemistry (2512)
Important MCQs Based on Medical Entrance Examinations To Improve Your NEET Score
18000+ students are using NEETLab to improve their score. What about you?
Solve Previous Year MCQs, Mock Tests, Topicwise Practice Tests, Identify Weak Topics, Formula Flash cards and much more is available in NEETLab Android App to improve your NEET score.
Share this page with your friends

Leave a Reply